DEHP Exposure
Diethylhexylphthalate (DEHP) is a phthalate, a chemical substance that is frequently used to soften PVC (Polyvinyl Chloride) medical devices, such as blood bags, tubing, catheters and disposable gloves to make materials more pliable and comfortable to use. The increasing knowledge of the effects of phthalate exposure, which can be teratogenic, carcinogenic or induce reproductive toxicity, has led to the development of alternative plasticisers.
PVC and Plasticizers
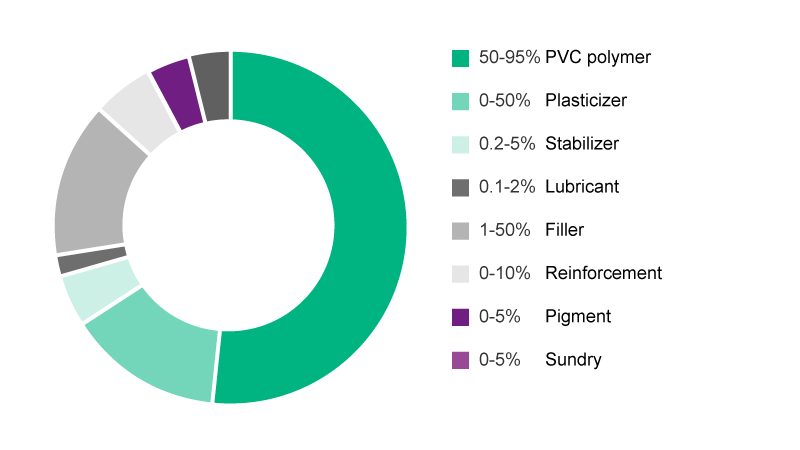
Polyvinyl chloride (PVC) plastic is used to manufacture a huge number of articles for daily life, e.g. toys, building material such as flooring, cables, as well as medical products.1 Unplasticized PVC is hard and brittle at room temperature. As a result, plasticizers are necessary to impart flexibility to the polymer. Plasticizers are additives, most commonly phthalate ester, which work by embedding themselves between the chains of polymers, spacing them apart, and thus significantly making it softer. For plastics such as PVC, the more plasticizer added, the more flexible and durable it will be (see Fig. 1 for the average content of substances in PVC).2
Besides di-(2-ethylhexyl) phthalate (DEHP), most commonly-used plasticizers today are phthalates, first and foremost, the following:
- DIDP (di-isodecyl-phthalate)
- DINP (di-isononyl-phthalate)
- DBP (dibutylphthalate)
- BBP (butylbenzylphthalate)
In addition to phthalates, there are also non-phthalates available on the market, although their current market share is only 8-10%. These include adipates, citrates, phosphates, trimellitates, etc.
Common alternatives include TOTM and Hexamoll DINCH, as well as the newly developed DEHT/DOTP (DEHT = Di(2-ethylhexyl) terephthalate resp. DOTP = Dioctyl terephthalate).
Various plasticizers have been used as plasticizers for PVC. The plasticizer of choice for PVC medical devices is DEHP.3
The content of DEHP in flexible polymer materials varies widely but is often around 30%-35% (w/w). Contrary to that, polyethylen and polypropylen normally do not contain any plasticizers.4,5
DEHP
DEHP is not known to occur naturally.
The worldwide production of DEHP has been increasing during recent decades. PVC is the second largest commodity plastic after polyethylene with world production currently over 18 million tonnes a year. The chemical process for making PVC involves three steps: first, production of the monomer, vinyl chloride; then the linking of these monomer units in a polymerisation process; and finally the blending of the polymer with additives.6
The industrial use and end-use of DEHP can be divided into three main product groups1:
I) PVC
II) non-PVC polymers
III) non-polymers
Around 97 % of DEHP is used as plasticizer in polymers, mainly PVC.
Some examples of flexible PVC end products containing DEHP are
- Insulation of cables and wires
- Profiles, hoses
- Sheets, film, wall- and roof covering and flooring
- Coatings and leather imitations (car seats, home furniture), shoes and boots, out-door and rainwear
- Pastes for sealing and isolation and Plastisols e. g. car undercoating
- Toys and child-care articles (pacifiers, teething rings, squeeze toys, crib bumpers etc.)
- Medical products
DEHP is used among other plasticizers as an additive to rubbers, latex, mastics and sealant, inks and pigments, lubricants.1
Some examples of non-polymer end products containing DEHP:
- Lacquers and paints
- Adhesives and Fillers
- Printing inks
- Dielectric fluids in capacitors
- Ceramics
PVC and DEHP in medical products
The use of PVC in medical devices represents a very minor percentage of the total amounts of PVC manufactured each year. Nonetheless the use of plasticized PVC in a wide range of medical devices has been very important for a number of reasons7:
- Flexibility in a variety of physical forms from tubes to membranes
- Chemical stability and possibility to sterilise
- Low cost and wide availability
- Lack of evidence of significant adverse consequences in patients
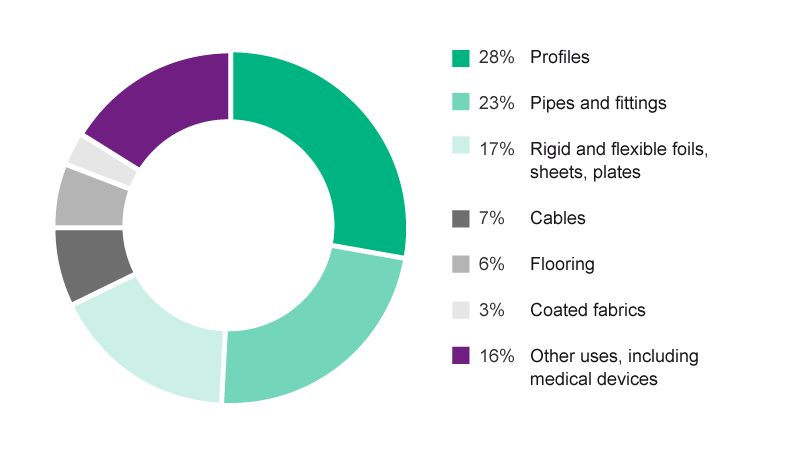
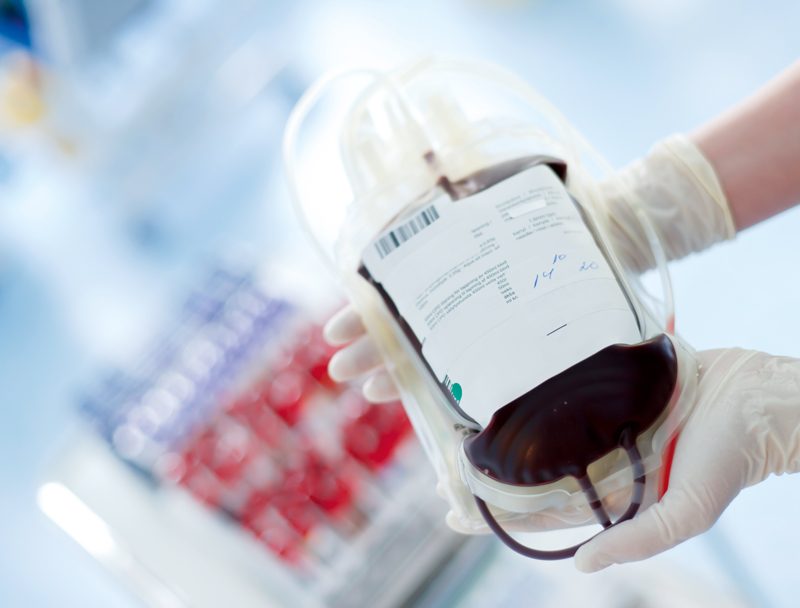
Approximately 3×104 tons of plasticized PVC is used for medical applications annually in Europe7, such as IV and blood bags and infusion tubing, enteral and parenteral nutrition feeding bags, and tubing used in devices for cardiopulmonary bypass and extracorporeal membrane oxygenation.
Did you Know?

(1) SCENIHR Opinion on the safety of medical devices containing DEHP plasticized PVC or other plasticizers on neonates and other groups possibly at risk (2008).
Causes
Especially with IV fluid containers made of DEHP containing PVC, there are three main effects of concern:
Leaching of DEHP
Everyone is exposed to small levels of DEHP in day to day life. However, some individuals can be exposed to high levels of DEHP through certain medical procedures. DEHP can leach out of plastic medical devices into solutions that come in contact with the plastic.
The amount of DEHP that will leach out depends on the temperature, the lipid content of the liquid, and the duration of contact with the plastic. Seriously ill individuals often require more than one of these procedures, thus exposing them to even higher levels of DEHP17. On a weight basis, DEHP may constitute 30–40 % of a typical blood bag.18 Jaeger and Rubin reported the leaching of DEHP from PVC blood bags into stored blood components; their data suggest a leaching rate of 0.25 mg DEHP/100 ml/day for whole blood stored at 4°C.19 For a blood transfusion in adults, a DEHP exposure of 600 mg has been reported.20
For platelet concentrate stored in blood bags, a leaching of DEHP has been quantified in the stored platelets. It was estimated that each patient received a total of 26.4 to 82.4 mg DEHP for 5 bags.18
Because infusion of platelets requires typically 30 min, and assuming a linear leaching rate of DEHP with time, the tubing involved in administering platelets might contribute at most 1.0 mg, a minor contribution which may be ignored.21
Others have reported considerable amounts of DEHP leaching into infusion solutions stored in IV PVC bags, such as parenteral nutrition22, cytostatics15 or antibiotics.23,24
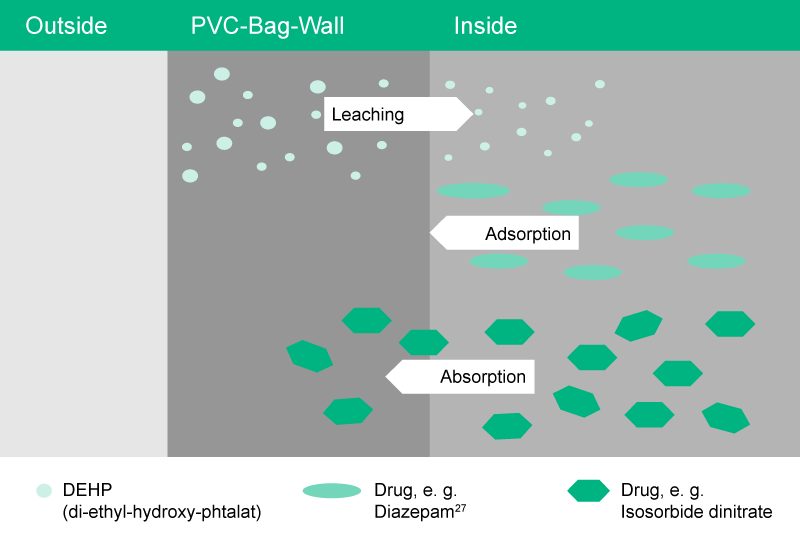
Sorption is a physical and chemical process by which one substance becomes attached to another.
Specific cases of sorption are:
- Leaching – Substance from bag wall migrates into the solution15
- Adsorption – Drug from solution, e. g. Diazepam16, is bound to the inner bag wall
- Absorption – Drug from solution, e. g. Isosorbide dinitrate, migrates into the bag wall25
Environmental exposure
Regarding the environment, two aspects have to be taken into consideration: One being the amount of DEHP which is released from plastics during or after the product lifetime, the other being side products during production and destruction of PVC.
Release of DEHP into the environment
Large amounts of DEHP in polymers are building up in:
- End products with long service lives (e. g. building material)
- Land fills
- Waste remaining in the environment (pieces of polymer)
- DEHP is assumed to be persistent as long as the molecule remains in the polimer matrix. Due to this high persistency the amount of DEHP in the technosphere (incl. the waste) is still increasing. The overall distribution of DEHP is 2% to air, 21% to water and 77% to urban/industrial soil.1
Side products of production and destruction of PVC
During production and especially incineration of PVC, a number of toxic by-products is released into the environment: Polychlorinated dibenzodioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and dioxin-like polychlorinated biphenyls (PCBs) are a group of structurally related chemicals that persist in the environment and may bioaccumulate in animal sources of food and in human tissues.30 Their half-life time in the body is estimated to be seven to eleven years.31
Example: Ciprofloxacin
The labeling information for Cipro® IV (Bayer AG, INN:ciprofloxacin) points out that DEHP can leach from the PVC container used to store and deliver this drug at a concentration up 5 parts per million (ppm). To estimate the dose of DEHP received by patients being administered this drug, information is needed on the volume of solution containing the drug and the frequency with which it is administered. For mild urinary tract infections, the recommended dose of ciprofloxacin is 200 mg every 12 hours. The drug comes packaged in a flexible PVC container that contains 200 mg in 100 ml of solution. If the concentration of DEHP in solution is 5 ppm or 5 mg/L, the dose of DEHP received by a patient receiving this drug to treat a urinary tract infection would be: 5 mg DEHP/L × 100 ml/ administration × 2 administrations/day × 0.001 L/ml = 1 mg DEHP/ day. More aggressive treatment is required to treat more severe infections. For example, a dose of 400 mg of ciprofloxacin is recommended every 8 hours to treat severe infections of the respiratory tract, bones, joints, or skin. The dose of DEHP received in this dosing regimen would be:
5 mg DEHP/L × 200 ml/administration × 3 administrations/day × 0.001 L/ml = 3 mg DEHP/day.
Example: Multiple drug infusions
Often, multiple drugs are co-infused in the same IV infusion. One such case is the co-infusion of quinine along with multivitamin preparations. It has been shown that little DEHP is released from PVC bags containing quinine alone in solution; however, the presence of the lipophilic multivitamin cocktail dramatically increased the extent of DEHP release from the bag. Following storage of quinine/multivitamin combinations for 48 hours at 45°C, the concentration of DEHP in the bags reached 21 mg/ml. Consequently, a patient receiving a 500 ml infusion of quinine with a multivitamin cocktail would receive 10.8 mg of DEHP. Non-PVC bags are available for the administration of drugs that require a lipophilic vehicle for solubilization. No DEHP is expected to be released from non-PVC bags. Consistent with this expectation, it was demonstrated that no DEHP was detected in a solution of paclitaxel stored in a polyethylene container for 15 days.17
Health Consequences
Health concerns about phthalate plasticizers are currently the subject of considerable media, legislative and scientific debate. Academia and industry have continually worked together to address the concerns and conduct necessary research, making phthalates some of the most researched and best understood chemicals today.32
Consequences of Leaching of DEHP
Negative effects of DEHP on human beings
Numerous studies have shown that the chemical group of the phthalates and especially DEHP impair testicular testosterone production in the rat. Very recent investigations have presented proof that DEHP can inhibit testosterone production in the adult human testis.33
Furthermore, adverse reproductive system outcomes, including reduced semen quality and altered male genital development, have been reported.34
In support of that, many phthalates are identified as anti-androgenic endocrine disrupting chemicals in mammalian models.35
Endocrine disrupting compounds are chemicals that can alter hormonal signaling with potential effects on developing reproductive and nervous systems, metabolism, and cancer.35
Serious concerns have been raised on exposure of ill newborns and neonates to DEHP.36Premature neonates in intensive care units, being dependent on multiple medical procedures, can receive even higher DEHP exposures than adults relative to their body weight. This exposure may be even higher than the doses observed to induce reproductive toxicity in animals.37
Negative effects on developing fetuses
Animal studies have shown DEHP to be particularly harmful to developing fetuses leading to adverse effects in the reproductive system, including changes in the testes.33,34

Fig. 7: Conclusion of the “National Toxicology Program” regarding the possibilities that human development or reproduction might be adversely affected by exposure to DEHP.41
Pregnant women exposed to high levels of phthalates may have increased risk of having sons with malformations of the genitals (hypospadias and cryptorchidism), low sperm count and increased risk of testis cancer.34,38
Carcinogenicity of DEHP
DEHP has been reported to be carcinogenic in liver in rats and mice with routes of induction being well investigated.39 Other adverse effects on lung, heart and kidney have been reported as well.40 The International Agency for Research on Cancer, part of the WHO, classified DEHP as possibly carcinogenic to humans (Group 2B)39, an opinion which has been adopted by many others, e. g. the US department of Health and Human Service.41
DEHP was downgraded by IARC in 2000, but massive criticism has been raised in the medical scientific community that IARC disregarded significant reports.
Lipophilic substances are of greatest concern
DEHP diffuses into lipophilic tissues and fluids and is thus distributed in the body, the route of ingestion, be it oral, parenteral, per inhalation or dermal, will not make a difference. Therefore, many authors advise the use of polyethylene or polypropylene containers rather than PVC15, and an increasing number of manufacturers of pharmaceuticals exclude the use of PVC bags for their drug preparations, e. g. for paclitaxel or temsirolimus.
Thrombogenicity of DEHP
PVC materials are well known to be of thrombogenic nature and there is substantial evidence that the extent of platelet aggregation is due to the presence of DEHP in the material and not the PVC itself. In addition, complement activation, a process associated with adverse hematological effects, is greater following exposure of blood to DEHP-plasticized PVC than to other polymers. Each of these effects can have adverse clinical consequences in patients.43,44
Peritoneal sclerosis associated with DEHP
Peritoneal sclerosis is a serious complication of peritoneal dialysis therapy. Beneath other factors, DEHP seems to have a role in the pathogenesis of this condition. Research results suggest that levels of DEHP in dialysate stored in DEHP bags are sufficient to initiate the process of peritoneal sclerosis and to produce sclerosis.
The clinical significance of peritoneal sclerosis cannot be underestimated, because patients with reduced dialytic capacity of the peritoneal membrane must be switched to hemodialysis.17
Consequences of sorption of PVC
Sorption of drugs to PVC and consequences for therapy
Whereas the discussion of leaching of plasticizers is focused on the toxicological properties of a drug packaging system, the sorption (superordinate of absorption and adsorption) of drug formulation compounds has an influence on the dosage of the active pharmaceutical ingredient resulting in a reduced drug delivery to the patient. Therefore, sorption has an influence on the effectiveness and success of the therapy.27
A list of drugs which are incompatible with PVC is given in Fig. 6. Considering these examples and their intended uses, the conceivable consequences result from significant underdosage of the substances:
- Underdosing of carmustine, an anti-cancer agent used for chemotherapy in glioblastoma (a brain tumor), might lead to non-effectiveness of the therapy and thus progression of cancer
- Underdosing of heparin or warfarin, both anti-coagulating drugs, might result in blood clotting and / or lung embolism
- Underdosing of thiopenthal, a rapid-onset short-acting barbiturate general anaesthetic, might result in unintended awareness of the patient
- Underdosing of isosorbide dinitrate and nitroglycerin, both dilating agents being used during angina pectoris, might be useless and angina pectoris might result in cardial infarction
- Chlordiazepoxide and diazepam are both sedatives and anxiolytics, might not be as effective as intended
In case the effect of sorption is known to the user and thus the amount of drug given is increased, this results in considerable preventable additional cost to the provider and payer.
Consequences from Environmental Exposure
The effects of DEHP leaching into the environment are the same as described above: via accumulation in the chain of food, they accumulate and reach the human body. Possible results may be endocrine disruption, cancer and a number of other malformations and diseases.
The effects of dioxins and furans released into the environment during production and incineration of PVC have been shown to exert a number of toxic responses, including dermal toxicity, neuro-developmental deficits, immunotoxicity, reproductive effects and teratogenicity, endocrine disruption, metabolic syndrome and carcinogenicity.30
In 1997, the International Agency For Research on Cancer classified TCDD (2,3,7,8-tetrachlorodibenzop-dioxin), most toxic compound of the group, as a group I carcinogen (sufficient evidence of carcinogenicity)45 and a recent review of both existing and new evidence supports this decision.46
As described above („Causes“), the amount of DEHP from building material, waste and landfills is continuing to increase in air, soil, and water. This environmental exposure adds to the exposure from food, medical devices and others and thus the described risks.
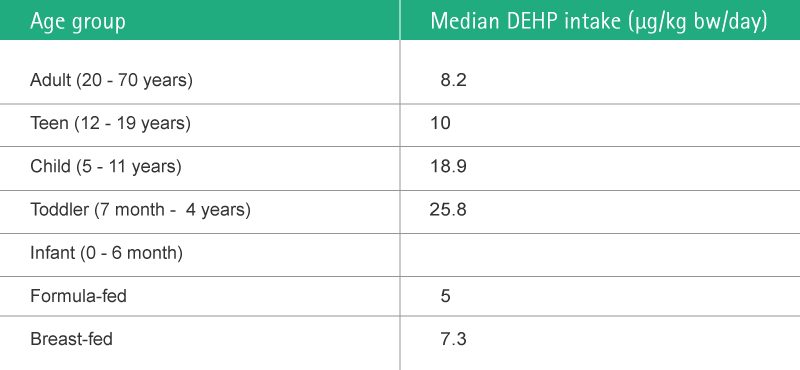
The dioxins and furans, toxic side products released by the production and destruction of PVC, (e. g. PCDDs, PCDFs and PCBs) are characterized by very long half-life times. Concentrations increase as they move up the food chain, mainly in fatty tissue. The median DEHP intake per age group is shown in Fig. 8.
Dioxins and furans are some of the most toxic chemicals known to science. Short-term exposure of humans to high levels of dioxins may result in skin lesions, such as chloracne and patchy darkening of the skin, and altered liver function. Long-term exposure is linked to impairment of the immune system, the developing nervous system, the endocrine system and reproductive functions.
Chronic exposure of animals to dioxins has resulted in several types of cancer. TCDD was evaluated by the WHO’s International Agency for Research on Cancer (IARC) in 1997.47 Based on animal data and on human epidemiology data, TCDD was classified by IARC as a „known human carcinogen”.
The health effects from exposure to dioxins and furans have been documented intensively in epidemiologic and toxicological studies.48 As well, a number of significant consequences are known from serious accidents such as the disaster in Seveso, Italy, in 1976. A cloud of toxic chemicals, including TCDD, was released into the air and eventually contaminated an area of 15 square kilometers where 37,000 people.
Financial Consequences
The exposure of human beings and especially developing children to DEHP can have significant health consequences. These disorders lead to significant economic consequences with more severe diseases leading to high economic burdens.
Potential Risk Associated Cost
As an example, some of the health consequences to DEHP have been selected and their additional treatment costs according to reports from the current literature are displayed below (see Fig. 9). These figures only take into account pure costs for treatment. Not included are any overall economic effects such as loss of labour, gross domestic product, joblessness, etc
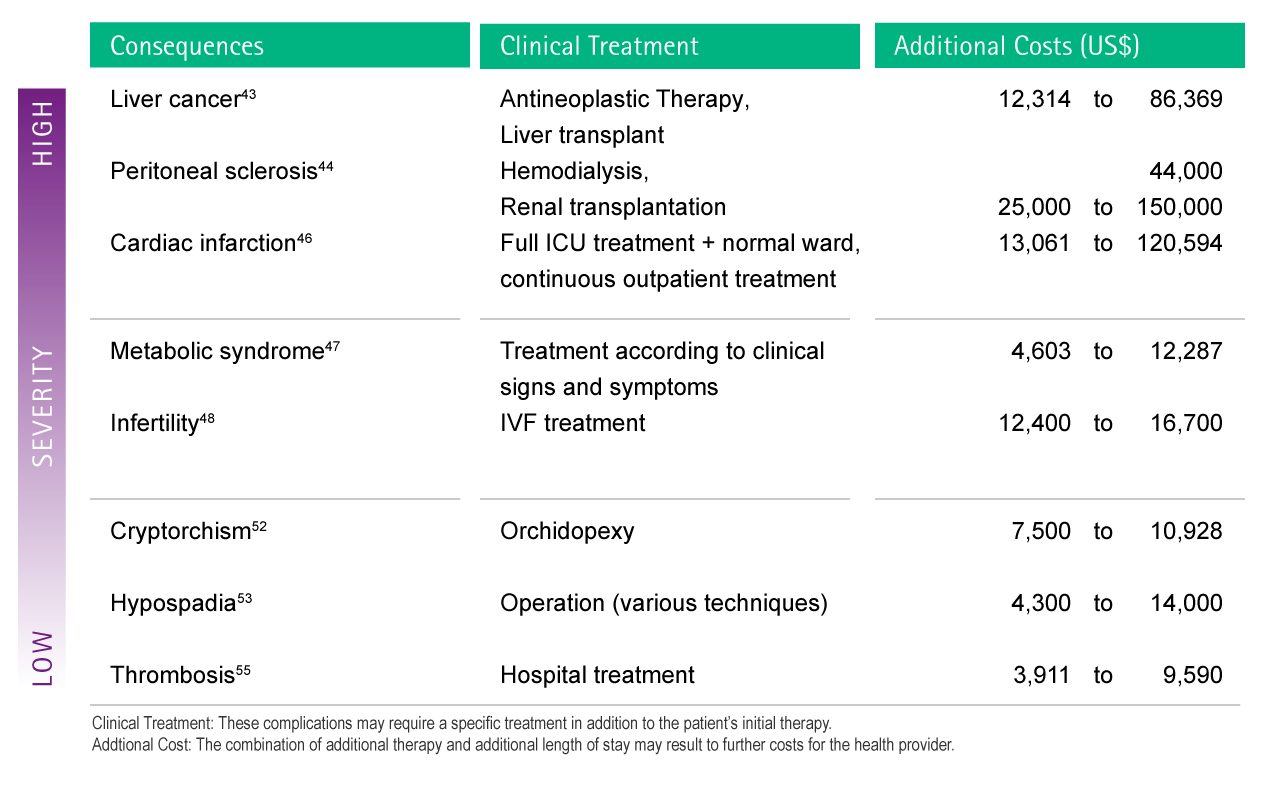
Fig. 9 : Estimation of possible additional costs as a consequence of complications caused by DEHP exposure. In order to facilitate attribution of each complication to the cost calculation, severity levels were introduced.79,80,81,82,83,84,85,86
Preventive Strategies
As outlined in the previous chapters, there is common agreement that the use of DEHP as plasticizer is of concern and scientific panels throughout the world recommended to limit its use. 1,4,7,17,49,50 Furthermore, there are nowadays a number of alternatives available, either using other plasticizers for PVC or completely different PVC-free materials. A number of international, national and regional activities have resulted from that, partly with legislative action, in the health care sector as well as in a number of other industries, such as toys or food and beverages.
Laws and Guidelines
- The German Federal Institute for Drugs and Medical Devices (BfArM) recommends using alternative products to DEHP-softened PVC medical devices for premature infants and new borns. BfArM also urges medical devices manufacturers to label products containing DEHP and put more effort into developing safer alternatives.58
- Concerns about dioxin from garbage incineration led the Japanese government to enact a new container and wrapping materials law requiring producers to recycle waste products by the year 2000. The law prompted several major Japanese makers of household goods and cosmetics to announce timetables by which they would switch to polypropylene and other materials for various types of cosmetic, food and pharmaceutical packaging.61
- The European Union recommends the use of other materials instead of DEHP-PVC for medical devices.51
- Similarly, the US Food and Drug Administration has issued an FDA Safety Assessment and a Public Health Notification urging health care providers to use alternatives to DEHP-containing devices for certain, vulnerable patients.17
- The latest draft of the guideline 2002/95/EG RoHS on the restriction of the use of certain hazardous substances in electrical and electronic equipment, the European Union has included DEHP into the list of prohibited substances.
- Argentina, Austria, Cyprus, the Czech Republic, Denmark, Fiji, Finland, Germany, Greece, Italy, Japan, Mexico, Norway, and Sweden have restricted phthalates in children’s toys.52
- The Health Care Without Harm campaign is an international coalition of 420 organisations in 51 countries; organisations include hospitals and health care systems, medical professionals, community groups, health affected constituencies, labor unions, environmental and health organisations and religious groups. One of the goals of the campaign is to phase out the use of PVC and persistent toxic chemicals, and to build momentum for a broader PVC phase out campaign.64
- Since 1st April 2005 cosmetic products containing DEHP shall not be supplied to consumers in the EU, in accordance with Commission Directive 2004/93/EC of 21st September 2004 amending Council Directive 76/768/EEC concerning cosmetic products.
- For food, PVC packaging which may or may not contain DEHP, has been either banned or restricted in a number of countries, including Canada, Spain, South Korea and the Czech Republic.57
- The European Community’s regulation EC 1907/2006 "Registration, Evaluation, Authorisation and Restriction of Chemical substances" (REACH) came into force on June 1st, 2007. It classifies DEHP as substance of Very High Concern. Such substances are subject to authorization through the European Chemicals Agency (ECHA) in Helsinki.54
- According to EC.directive 2007/47, medical devices containing DEHP have to be labelled accordingly.55
- With regards to food packaging, the use of DEHP in food contact materials is already restricted under Commission Directive 2007/19/EC of 30 March 2007 relating to plastic materials and articles intended to come into contact with food and Council Directive 85/572/EEC laying down the list of simulants to be used for testing migration of constituents of plastic materials and articles intended to come into contact with foodstuffs.
- Health Canada’s scientific expert panel on DEHP recommended among others that storage bags used for the administration of lipophilic drugs, should not contain DEHP23. As well, Health Canada has proposed to prohibit the sale, advertisement, and importation of toys for children under three years of age and products for children under three years of age that are likely to be mouthed and contain DEHP.59
- Effective August 2008, the United States Congress signed the Consumer Product Safety Improvement Act (CPSIA) in which section 108 specified that as of February 10th, 2009, it is unlawful to manufacture for sale, offer for sale, distribute in commerce, or import any children’s toy or child care article that contains concentrations of more than 0.1 % of DEHP, DBP, or BBP.
- In January 2010, the Australian Consumer Affairs Minister Craig Emerson announced a ban on items containing more than 1% DEHP because of reproductive difficulties.56
- The requirements of the Stockholm Convention for releases of dioxins and other by-product Persistent Organic Pollutants (furans, hexachlorobenzene and PCBs) are that each party shall, at a minimum reduce the total releases derived from anthropogenic sources of each of the chemicals … with the goal of their continuing minimization and, where feasible, ultimate elimination.60
- In the EU-regulation No. 143/2011, bis(2-ethylhexyl)phthalate (DEHP) is classified as toxic to the reproductive system. From January 21st 2015 onwards, the placing on the market and the use of DEHP without special permission will be prohibited.53
Initiatives and Campaigns
- Kaiser Permanente, Miller Children‘s Hospital in Long Beach, Lucile Packard Children‘s Hospital at Stanford University, and John Muir Medical Center in Walnut Creek are phasing out PVC medical devices from NICUs.64
- In Spain, over 60 municipalities have approved PVC phase-out measures. The same applies to Germany, where an increasing number of cities approved by their own statements avoid PVC in public buildings.52
- The Confederation for Environmental and Nature Conservation Germany (Bund für Umwelt und Naturschutz Deutschland), Health Care Without Harm (HCWH) and the European Academy for Environmental Medicine (EUROPAEM) have started the initiative "pollution-free hospital". The environmental organizations have called on the hospitals in Germany to forego PVC-containing medical devices.65 The Pediatric Clinic Glanzing of the Vienna Hospital Association is the first Neonatology Unit world wide to announce it will manage completely without PVC plastic.64
- In November 2011, the Food and Drug Administration (FDA) has revealed a new maximum allowable level (0.006 mg/l) for diethylhexyl phthalate (DEHP) in bottled water and manufacturers are required to monitor the levels.62
- The OSPAR List of Chemicals for Priority Action includes several substances which are by-products of the production of chlorine and PVC, or additives in PVC: dioxins & furans, chlorinated paraffins, mercury and organic mercury compounds, lead and organic lead compounds, organic tin compounds, certain phthalates (DBP & DEHP).66
- The managed health-care provider Kaiser Permanente said it will no longer purchase intravenous solution bags made from PVC or that contain the plasticizer as part of its continuing effort to better protect the health and safety of the 8.9 million people who get care at its hospitals, doctors’ offices and health-care facilities.63
Research and Industry
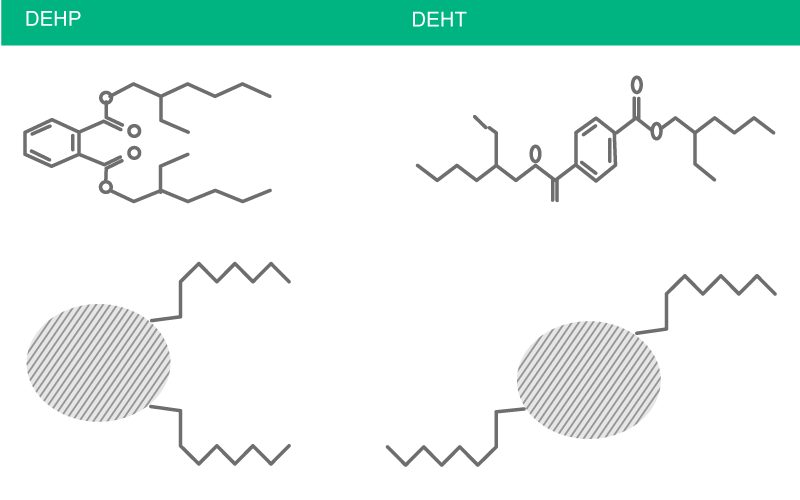
Fig. 10: DEHT means Di-(2-ethylhexyl)-terephthalate, also named DOTP (Dioctylterephthalate). The chemical structure is not identical to the one of DEHP.
- B. Braun has bought and further developed Di(2-ethylhexyl) terephthalate resp. Dioctyl terephthalate (DEHT or DOTP, resp.), a non-phtalate plasticizer with a different chemical structure. DEHT or DOTP is the only plasticizer known for flexible PVC which does not show any toxic side effects in tests (see Fig. 10).7, 67
- Alternative plasticizers have been developed and manufactured by a growing number of chemical manufacturers. Comprehensive and thorough research is necessary before these plasticizers will be approved, such as TOTM/TEHTM and Hexamoll®DINCH.
- A number of medical device companies including B. Braun have invested into research on other materials than DEHP-PVC for medical devices.
Characteristics of the alternative plasticizer DEHT
Di(2-ethylhexyl) terephthalate (DEHT) is a general purpose non-phthalate plasticizer which is in commercial use since 1975. For example, Coca-Cola and other companies have used DEHT ever since for their bottles. Terephthalate is the „T“ in PET. As well, the toy industry heavily uses this plasticizer.
DEHT has been invented and produced by the Eastman Company, thus, one of its trade names is Eastman 168.
Toxicity profile of DEHT
DEHT has been studied in a wide variety of in vitro and in vivo studies on its toxicity profile. Acute toxicity studies on oral, dermal, and ocular exposure as well as inhalation have been conducted, with focus on acute, sub-acute and sub-chronic toxicity. All studies have shown an excellent toxicological profile.
Developmental toxicity tests revealed neither an alteration of male rat sexual differentiation during development nor an effect on male organ development. There was no estrogenic effect either. Tests for genotoxicity and mutagenicity were all negative. There was no effect upon tumor incidence and tumorigenicity and there was no induction of liver peroxisomes, which are regarded as sign for potential tumorigenicity.
A GLP-conform toxicity study in male and female rats with continuous intravenous infusion of DEHT over 4 weeks showed that DEHT administered via IV infusion was tolerated systemically and locally without adverse effects up to and including 381.6mg/kg/day (NOAEL=381.6mg/kg×day). In particular, there were no effects on reproductive tissues/organs, kidneys, liver hepatocytes and peroxisomes, as known targets of DEHP-toxicity. A clinical study on 203 volunteers was conducted in order to test DEHT on its irritation skin potential.68 DEHT was concluded to not be irritating, and did not induce contact sensitization.
These data indicate that DEHT (Eastman 168) plasticizer has a low order of acute toxicity, is essentially non-irritating, and is not likely to induce contact sensitization in humans.69
Regulatory profile of DEHT
DEHT Eastman 168 has been thoroughly evaluated by several government agencies around the world and placed on approved lists.
USA: recognized by the Food Contact Notification (FCN) for a Variety of Food Contact Applications.70
European Union: European Food Safety Authority (EFSA) approval; SCENIHR review for use in medical applications. DEHT is not classified as CMR (carcinogenic, mutagenic or toxic to reproduction) by REACH.54 Products not containing DEHP can be marked as DEHP-free with appropriate symbols.
Germany: approved for use in plasticized PVC including beverage tubing, cap liners, and food wrap by the “Bundesinstitut für Risikobewertung (BfR), (German Institute for Risk Assessment).
Switzerland: DEHT is not listed on Swiss toxic list, thus not considered as toxic.
Japan: Japan Hygienic PVC Association includes DEHT on its positive list.
Environmental profile
The solubility of DEHT in water is very low. In distilled water the solubility has been reported to be 0.4 μg/l. The aquatic toxicity data indicates that there were no acute or chronic effects for any species tested at concentrations at or near the water solubility limit of the material. One study also indicated no impact on survival at concentrations that were orders of magnitude above the solubility limit for the test conditions. Sub-lethal effects such as growth, reproduction, shell deposition, and egg hatchability were also not adversely impacted in any of the studies at the concentrations tested. Because DEHT would have a strong tendency to sorb to sediments in the aquatic environment, an OECD sediment-water chironomid toxicity test using spiked sediments was conducted to demonstrate that DEHT would not have cause adverse impacts to aquatic sediment dwelling organisms. The sediment test indicated that the EC50 was greater than the highest test concentration recommended by the method.
With regards to biodegration, studies have shown that 37-56 % of the original DEHT was degraded in 28 days.
These studies indicate that DEHT is susceptible to both ultimate and primary degradation.69
Further material for medical devices and drug containers
Bearing in mind the shortcomings of PVC, especially the sorption effects having an influence on the effectiveness and success of the therapy, other materials for medical devices and drug containers have been subject to research. More inert polymers like polyethylene terephthalate (PET) or polyamide (PA) result in a lower interaction.71,72 On the other hand, not every polymer is suitable for packaging of pharmaceuticals. Due to its rigid mechanical properties, PET for example can hardly be used for flexible tubes or bags.27
Considerations like these have also led to compound materials such as multi-layer foils, where the inner layer is made of polypropylene and further outer layers consist of polyethylene and polyester.
Sorption behaviour of polyethylene / polypropylene
Polyethylene (PE) and polypropylene (PP), both belonging to the class of polyolefines, are two such materials which have been proven suitable for manufacturing of IV containers. Following the European Pharmacopoeia73 drug containers made from medical grade PE are free from plasticizers, additives and other compounds that may potentially migrate into the finished preparation. They are chemically inert and toxicologically safe.
As representative examples for the well known group of drugs exhibiting significant sorption to PVC, nitroglycerin and diazepam have been investigated for their sorption behaviour towards alternative plastics. For both drugs, PE and PP showed significantly lower sorption rates than standard PVC tubes.27
A number of further studies have shown that PE/PP exert the lowest sorption potential on drugs, compared to other plastics.74,75,76
In general, modeling of the solution interaction properties of plastic materials used in pharmaceutical container systems has revealed that PP has the lowest binding propensity for drugs, followed closely by PE.77
Environmental effects of polyethylene / polypropylene
Most of the medical waste is nowadays subject to incineration. For example, in the UK, the “Landfill Directive” sets the rule that clinical waste may not be disposed on landfills, but needs to be incinerated. As outlined before, disposal of PVC via incineration is associated with the generation and dispersal of polychlorinated dibenzo[p]dioxins (PCDDs) and polycarbonated dibenzo-p-furans (PCDFs) into the environment, both of which are present in flue gases and ash.
Polyethylene and polypropylene, like all hydrocarbons, are burning very well. The only residues from complete combustion are carbon dioxide and water as combustion products, which are not toxic and do not pose any environmental risk.78
Highlight Safety Products
Highlight safety products
Scientific Evidence
1 European Union Risk Assessment Report. bis(2-ethylhexyl)phthalate (DEHP)
2 Cadogan DF, Howick CJ (2000)
Plasticizers in Ullmann’s Encyclopedia of Industrial Chemistry,
Wiley-VCH, Weinheim. doi: 10.1002/14356007.a20_439.
3 Safety Assessment of Di(2-ethylhexyl)phthalate (DEHP) Released from PVC Medical Devices
http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM080457.pdf
4 Umweltbundesamt, Berlin, Hrsg. (2/2007)
Phthalate – die nützlichen Weichmacher mit den unerwünschten Eigenschaften: 3, 10.
5 Kroschwitz JI (1998)
Kirk-Othmer Encyclopedia of Chemical Technology.
Fourth Edition. John Wiley and Sons, New York.
6 http://www.chm.bris.ac.uk/webprojects2001/esser/manufacture.html
7 SCENIHR opinion on the safety of medical devices containing dehp plasticized pvc or other plasticizers on neonates and other groups possibly at risk 2008.
8 Haishima Y, Seshimo F, Higuchi T, Yamazaki H, Hasegawa C, et al. (2005)
Development of a simple method for predicting the levels of di(2-ethylhexyl) phthalate migrated from PVC medical devices into pharmaceutical solutions.
Int J Pharm 2005; 298:126-42.
9 Hanawa T, Muramatsu E, Asakawa K, Suzuki M, Tanaka M, et al. (2000)
Investigation of the release behavior of diethylhexyl phthalate from the polyvinyl-chloride tubing for intravenous administration.
Int J Pharm; 210:109-15.
10 Hanawa T, Endoh N, Kazuno F, Suzuki M, Kobayashi D, et al. (2003)
Investigation of the release behavior of diethylhexyl phthalate from polyvinyl chloride tubing for intravenous administration based on HCO60.
Int J Pharm; 267:141-9.
11 Loff S, Kabs F, Witt K, Sartoris J, Mandl B, Niessen KH, Waag KL (2000)
Polyvinylchloride infusion lines expose infants to large amounts of toxic plasticizers.
J Pediatr Surg; 35:1775-81.
12 Loff S, Kabs F, Subotic U, Schaible T, Reinecke F, Langbein M (2002)
Kinetics of diethylhexylphthalate extraction from polyvinylchloride-infusion lines.
JPEN J Parenter Enteral Nutr; 26:305-9.
13 Loff S, Subotic U, Reinicke F, Wischmann H, Brade J (2004)
Extraction of Di-ethylhexyl-phthalate from Perfusion Lines of Various Material, Length and Brand by Lipid Emulsions.
J Pediatr Gastroenteral Nutr; 39:341-345.
14 Bourdeaux D, Sautou-Miranda V, Bagel-Boithias S, Boyer A, Chopineau J (2004)
Analysis by liquid chromatography and infrared spectrometry of di(2-ethylhexyl)phthalate released by multilayer infusion tubing.
J Pharm Biomed Anal; 35:57-64.
15 de Lemos ML, Hamata L, Vu T. (2005)
Leaching of diethylhexyl phthalate from polyvinyl chloride materials into etoposide intravenous solutions.
J Oncol Pharm Pract;11(4):155-7
16 Trissel LA, Pearson SD (2/1994)
Storage of lorazepam in three injectable solutions in polyvinyl chloride and polyolefin bags.
Am J Hosp Pharm; 1;51(3):368-72.
17 FDA Public Health Notification: PVC Devices Containing the Plasticizer DEHP.
18 Rubin RJ, Schiffer CA (1976)
Fate in humans of the plasticizer, di-2-ethylhexyl phthalate, arising from transfusion of platelets stored in vinyl plastic bags.
Transfusion; 16(4): 330–335.
19 Jaeger RJ, Rubin RJ (1972)
Migration of a phthalate ester plasticizer from polyvinyl chloride blood bags into stored human blood and its localization in human tissues.
N Engl J Med; 287(22): 1114–1118.
20 Sjöberg P, Bondesson U, Sedin G and Gustafsson J (1985)
Disposition of di- and mono- 2ethylhexyl)phthalate in newborn infants subjected to exchange transfusions.
Eu J Clin. Invest; 15: 430-436.
21 Easterling RE, Johnson E, Napier EA (1974)
Plasma extraction of plasticizers from “medical grade” polyvinylchloride tubing.
Proc Soc Exp Biol Med 147: 572–574.
22 Bagel S, Dessaigne B, Bourdeaux D, Boyer A, Bouteloup C, Bazin JE, Chopineau J, Sautou V. (2011)
Influence of lipid type on bis (2-ethylhexyl)phthalate (DEHP) leaching from infusion line sets in parenteral nutrition.
JPEN J Parenter Enteral Nutr; 35(6): 770-5
23 Health Canada (2002) An exposure and toxicity assessment. Medical Devices Bureau, Therapeutic Products Directorate, Health Products and Food Branch, Ottawa, Canada.
24 Pearson SD, Trissel LA (1993)
Leaching of diethylhexyl phthalate from polyvinyl chloride containers by selected drugs and formulation components.
Am J Hosp Pharm; 50:1405-9.
25 Lee MG, Fenton-May V (1981)
Absorption of isosorbide dinitrate by PVC infusion bags and aministration sets.
J Clin Hosp Pharm; Sep;6 (3):209-11.
26 Beitz C, Bertsch T, Hannak D, Schrammel W, Einberger C, Wehling M (8/2005)
Compatibility of plastics with cytotoxic drug solutions—comparison of polyethylene with other container materials.
Int Journ of Pharm; (1)185: 113–121.
27 Treleano A, Wolz G, Brandsch R, Welle F (3/2009)
Investigation into the sorption of nitroglycerin and diazepam into PVC tubes and alternative tube materials during application.
Int J Pharm; 18; 369(1-2):30-7.
28 Martens HJ, De Goede PN, Van Loenen AC (2/1990)
Sorption of various drugs in polyvinyl chloride, glass, and polyethylene-lined infusion containers.
Am J Hosp Pharm; 1990 Feb;47(2):369-73.
29 Frenette AJ, MacLean RD, Williamson D, Marsolais P, Donnelly RF (9/2011)
Stability of levothyroxine injection in glass, polyvinyl chloride, and polyolefin containers.
Am J Health Syst Pharm; 15;68(18):1723-8.
30 Hedley AJ, Wong TW, Hui LL, Malisch R, Nelson EA (2/2006)
Breast milk dioxins in Hong Kong and Pearl River Delta.
Environ Health Perspect; 114(2):202-8.
31 WHO Factsheet no 225, 2010,
Dioxins and their effect on human health, May 2012
32 http://www.pvc.org/en/p/Health_concerns_about_Phthalate_plasticisers
33 Zhang LD, Li HC, Chong T, Gao M, Yin J, Fu DL, Deng Q, Wang ZM. (2014)
Prepubertal exposure to genistein alleviates di-(2-ethylhexyl) phthalate induced testicular oxidative stress in adult rats.
Biomed Res Int; 2014: 598630. doi: 10.1155/2014/598630.
34 Kay VR, Bloom MS, Foster WG. (2014)
Reproductive and developmental effects of phthalate diesters in males.
Crit Rev Toxicol; 44(6): 467-98.
35 Pflieger-Bruss S, Schuppe HC, Schill WB. (2004)
The male reproductive system and its susceptibility to endocrine disrupting chemicals.
Andrologia; 36(6): 337-45.
36 Mallow EB, Fox MA. (2014)
Phthalates and critically ill neonates: device-related exposures and non-endocrine toxic risks.
J Perinatol; 34(12): 892-7.
37 Pak VM, Nailon RE, McCauley LA. (2007)
Controversy: neonatal exposure to plasticizers in the NICU.
MCN Am J Matern Child Nurs; 32(4): 244-9.
38 Marie C, Vendittelli F, Sauvant-Rochat MP. (2015)
Obstetrical outcomes and biomarkers to assess exposure to phthalates: A review.
Environ Int; 83: 116-36.
39 Rusyn I, Corton JC. (2012)
Mechanistic considerations for human relevance of cancer hazard of di(2-ethylhexyl) phthalate.
Mutat Res; 750(2): 141-58
40 Tickner JA, Schettler T, Guidotti T, McCally M, Rossi M. (2001)
Health risks posed by use of Di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: a critical review.
Am J Ind Med; 39(1): 100-11.
41 National toxicology programm, the US department of health and human services (11/2006) Center for the evaluation of risks to human reproduction:
NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Di(2-Ethylhexyl) Phthalate (DEHP).
NIH Publication No. 06 – 4476.
42 Fachinformation Taxol (1/2009), Abschnitt 6.2. Brystol-Meyers-Squibb.
43 Panknin HT.
Particle release from infusion equipment: etiology of acute venous thromboses.
Kinderkrankenschwester. 2007;26:407-8.
44 Danschutter D, Braet F, Van Gyseghem E, Hachimi-Idrissi S, Van Bruwaene B, Moloney-Harmon P, Huyghens L.
Di-(2-ethylhexyl) phthalate and deep venous thrombosis in children: a clinical and experimental analysis.
Pediatrics. 2007;119:e742-53.
45 IARC (1997)
Polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans.
IARC Monogr Eval Carcinog Risks Hum 69:1–343.
46 Steenland K, Bertazzi P, Baccarelli A, Kogevinas M (2004)
Dioxin revisited: developments since the 1997 IARC classification of dioxin as a human carcinogen.
Environ Health Perspect 112:1265–1268.
47 IARC Working Group on the Evaluation of Carcinogenic Risks to Humans:
Polychlorinated Dibenzo-Para-Dioxins and Polychlorinated Dibenzofurans.
IARC Monogr Eval Carcinog Risks Hum. 1997;69:1-631.
48 EPA 2012
Reanalysis of key issues related to dioxin toxicity and response to NAS comments
49 Bernard L, Décaudin B, Lecoeur M, Richard D, Bourdeaux D, Cueff R, Sautou V; Armed Study Group. (2014)
Analytical methods for the determination of DEHP plasticizer alternatives present in medical devices: a review.
Talanta; 129: 39-54
50 Fischer CJ, Bickle Graz M, Muehlethaler V, Palmero D, Tolsa JF. (2013)
Phthalates in the NICU: is it safe?
J Paediatr Child Health;49(9):E413-9
51 European Commission, T. S. C. o. M. P. a. M. D. (2002).
“Opinion on Medical Devices Containing DEHP Plasticised PVC; Neonates and Other Groups Possibly at Risk from DEHP Toxicity.”
52 Greenpeace International (2003) PVC-Free Future:
A Review of Restrictions and PVC-free Policies Worldwide.
53 Änderung im Anhang der REACH-Regulation (PDF), Stand 17. Februar 2011
54 REACH - Registration, Evaluation, Authorisation of Chemicals (2007)
55 RICHTLINIE 2007/47/EG DES EUROPÄISCHEN PARLAMENTS UND DES RATES
vom 5. September 2007 zur Änderung der Richtlinien 90/385/EWG des Rates zur Angleichung der Rechtsvorschriften der Mitgliedstaaten über aktive implantierbare medizinische Geräte und 93/42/EWG des Rates über Medizinprodukte sowie der Richtlinie 98/8/EG über das Inverkehrbringen von Biozid-Produkten
56 Emerson C, ALP Australia Bans Phthalates in Toys.
57 PVC: The Poison Plastic. PVC Governmental Policies around the World.
58 Referenz-Nr.: 9211/0506 DEHP als Weichmacher in Medizinprodukten aus PVC.
Erstellt: 22.05.2006
59 Health Canada. Consumer Product Safety. (2007) Proposal for legislative action on di(2-ethylhexyl) phthalate under the Hazardous Products Act.
60 http://chm.pops.int
61 Japanese Makers to Switch from PVC to Eco-Based Containers’, Nikkei, Tokyo, January 14, 1998.
62 FoodBev.com, 31.01.2012
63 http://www.plasticsnews.com/headlines2.html?ncat=&id=24284
64 Health Care Without Harm (2003)
Glanzing Clinic in Vienna is First PVC-Free Pediatric Unit Worldwide.
Press release (June 13, 2003).
65 Initiative „Schadstofffreies Krankenhaus“ gegen PVC-haltige Medizinprodukte.
Deutsches Ärzteblatt, Ausgabe 15. November 2006;
66 OSPAR List of Chemicals for Priority Action (Revised 2011),
Reference number 2004-12;
67 Wirnitzer, U (2011)
Non-Clinical Testing of the Plasticizer Di-2-ethylhexyl-terephtalate (DEHT) under Clinically relevant Conditions.
ITO Symposium 15.-16. Sep 2011.
68 Wirnitzer U, Rickenbacher U, Katerkamp A, Schachtrupp A.
Systemic toxicity of di-2-ethylhexyl terephthalate (DEHT) in rodents following four weeks of intravenous exposure.
Toxicol Lett. 2011 Aug 10;205(1):8-14.
69 Unpublished data, Eastman Chemical Company.
70 https://www.accessdata.fda.gov/scripts/fdcc/?set=ENV-FCN
71 Stoffers, N.H., Stoermer, A., Bradley, E.L., Brandsch, R., Cooper, I., Linssen, J.P.H., Franz, R., 2004.
Feasibility study for the development of certified reference materials for specific migration testing: Part 1. Initial migration concentration and specific migration.
Food Addit. Contam. 21, 1203–1216.
72 Stoffers, N.H., Brandsch, R., Bradley, E.L., Cooper, I., Dekker, M., Stoermer, A., Franz, R., 2005.
Feasibility study for the development of certified reference materials for specific migration testing: Part 2. Estimation of diffusion parameters and comparison of experimental and predicted data.
Food Addit. Contam. 22, 173–184.
73 European Pharmacopoeia monograph 3.1.4 of the Polyethylene without additives for containers for preparations for parenteral use and for ophthalmic preparations.
74 Kaiser J., Krämer I.
Physicochemical stability of diluted trastuzumabinfusion solutions in polypropylene infusion bags.
Int J Pharm Compounding 2011 15:6 (515-520).
75 Menard C., Bourguignon C., Schlatter J., Vermerie N., Schlatter J.
Stability of Cyclophosphamide and Mesna Admixtures in Polyethylene Infusion Bags.
Annals of Pharmacotherapy 2003 37:12 (1789-1792).
76 Arsène M., Favetta P., Favier B., Bureau J., Favetta P.
Comparison of ceftazidime degradation in glass bottles and plastic bags under various conditions.
Journal of Clinical Pharmacy and Therapeutics 2002 27:3 (205-209).
77 Jenke D, Couch T, Gillum A, Sadain S.
Modeling of the solutioninteraction properties of plastic materials used in pharmaceutical product container systems.
PDA J Pharm Sci Technol. 2009 Jul-Aug;63(4):294-306.
78 Römpp Lexikon Chemie, 9. Auflage 1992
79 The Straits Times, Thu, Oct 29, 2009
80 The costs of myocardial infarction-a longitudinal analysis using data from a large German health insurance company Reinhold T, Lindig C, N. Willich S, Brüggenjürgen B. Journal of Public Health 2011 19:6 (579-586)
81 Nephrol Dial Transplant (2009) 24: 3209–3215. The Pan-Thames EPS study: treatment and outcomes of encapsulating peritoneal sclerosis. Gowrie Balasubramaniam, Edwina A. Brown, Andrew Davenport, Hugh Cairns, Barbara Cooper, Stanley L. S. Fan, Ken Farrington, Hugh Gallagher, Patrick Harnett, Sally Krausze and Simon Steddon.
82 Prevalence rates and costs of metabolic syndrome and associated risk factors using employees' integrated laboratory data and health care claims. Birnbaum H.G., Mattson M.E., Kashima S., Williamson T.E. Journal of Occupational and Environmental Medicine 2011 53:1 (27-33)
83 Health economics perspective of the components of the cardiometabolic syndrome Tamariz L., Palacio A., Florez H., Tamariz L., Palacio A., Yang Y., Parris D., Florez H., Tamariz L., Palacio A., Florez H., Ben-Joseph R.
84 Economic Analysis of Infant vs Postpubertal Orchiopexy to Prevent Testicular Cancer. Hsieh M.H., Roth D.R., Meng M.V. Urology 2009 73:4 (776-781)
85 Staged reconstruction of hypospadias with chordee: Outcome and costs. Svensson H, Reychman M, Åberg M, Troëng T, Svensson H. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery 1997 31:1 (51-55)
86 One-year costs in patients with a history of or at risk for atherothrombosis in the United States. Mahoney E.M., Wang K., Cohen D.J., Hirsch A.T., Alberts M.J., Eagle K., Mosse F., Jackson J.D., Steg P.G., Bhatt D.L., REACH Registry Investigators Circulation. Cardiovascular quality and outcomes 2008 1:1 (38-45)