Microbiological Contamination
Microbiological contamination refers to the non-intended or accidental introduction of infectious material like bacteria, yeast, mould, fungi, virus, prions, protozoa or their toxins and by-products.1,2
“A nosocomial infection — also called “hospital-acquired infection” is defined as:
An infection occurring in a patient in a hospital or other healthcare facility in whom the infection was not present or incubating at the time of admission. This includes infections acquired in the hospital but appearing after discharge, and also occupational infections among staff of the facility.”3
Types of microbiological pathogens
There is a broad range of microbiological pathogens, which can cause contamination and thus infections. Within these groups, several different types of pathogens exist:
Bacteria
Bacteria are microorganisms with a size of up to 5 µm and represent the most important group of pathogens when discussing microbiological contamination. According to the constitution of their cell wall, bacteria can be distinguished into Gram-positive and Gram-negative bacteria. Bacteria can be further distinguished as follows:
"Commensal" bacteria:
belong to the normal flora of healthy humans. They are usually harmless to healthy people or even have a significant protective role by preventing colonization by pathogenic microorganisms. Some commensal bacteria may however cause infection, if the natural host is compromised or if they are brought into the host’s tissue.3
Pathogenic bacteria:
have greater virulence and cause infections regardless of the host’s status.3
Viruses
Viruses are subcellular biological objects with a size of 20-200 nm. They exist with and without envelopes (shells mostly derived from host membranes covering the virus) and can cause serious infections.3
Prions
Prions are infectious protein particles. They are the smallest pathogens, which are below 5 nm in size.
Both prions and viruses are particles without own metabolism and are thus not regarded as living organisms. For reproduction, they depend on the metabolism of a host organism.3
Funghi, Yeasts and Protozoa
Fungi, yeasts and protozoa with up to 200 µm in diameter are three further groups of infection sources.3
Catheter-Related Blood Stream Infection (CR-BSI)
The definition of CR-BSI helps with the decision whether a catheter is the primary source of bacteremia in a patient. They include exit site or tunnel infections and are defined as:
- Erythema or induration within 2 cm of the catheter exit site, with or without concomitant bloodstream infection and curulence.
- For tunnel infections, presence of tenderness, erythema, or site induration >2 cm from the catheter site along the subcutaneous tract of a tunneled catheter in the absence of concomitant blood stream infection is required.5
Microbiological contamination is most dangerous for patients when it affects parenteral therapy and the intravenous catheters used. In this case, pathogens can directly reach the systemic circulation and cause catheter-related blood stream infection (CR-BSI) or travel to various organs and induce organ failure.
Therefore, prevention of CR-BSI is crucial. In the mid-90s the Centers for Disease Control and Prevention (CDC) published a standard definition for CR-BSI, which is the most widely accepted definition for CR-BSI.6
Bacterial infections can mostly be treated with antibiotic drugs. However, there are cases where this is extremely difficult or even impossible because the bacteria have become multidrug resistant. Against most viruses and all prion diseases, there are also no effective drugs available. Thus, prevention of such infections is crucial.
Methicillin resistant Staphylococcus aureus (MSRA) infections
Methicillin resistant Staphylococcus aureus (MRSA) infection is a serious worldwide health concern. MRSA is defined as any strain of Staphylococcus aureus that has developed resistance to beta-lactam antibiotics which include the penicillins (methicillin, dicloxacillin, nafcillin, oxacillin, etc.) and the cephalosporins.
According to the Centers for Disease Control and Prevention (CDC), MRSA currently causes about 1% of all staphylococcus infections and more than 50% of health-care associated staphylococcus infections. After Staphylococcus epidermidis, Staphylococcus aureus is the second most common pathogen causing health care-associated infections in the United States, and 49% of those infections are caused by the highly antibiotic resistant bacteria MRSA.9
A strain called USA100 is the most common type of MRSA involved in health care-associated infections in U.S. hospitals.8 MRSA is especially troublesome in hospitals and nursing homes where patients with open wounds, invasive devices and weakened immune systems are at greater risk of infection than the general public. Each year in the United States, more than 290,000 hospitalized patients are infected with Staphylococcus aureus. Of these staphylococcal infections, approximately 126,000 are related to MRSA.9
“Nosocomial infections are widespread. They are important contributors to morbidity and mortality. They will become even more important as a public health problem with increasing economic and human impact because of:
- Increasing numbers and crowding of people
- More frequent impaired immunity
(age, illness and treatments) - New microorganims
- Increased bacterial resistance to antibiotics.4
Multidrug resistant bacteria
Multidrug resistance is a condition enabling a disease-causing organism to resist distinct drugs or chemicals of a wide variety of structure and function targeted at eradicating the organism.10
Important multidrug resistant organisms are:
- Methicillin resistant Staphylococcus aureus (MRSA)
- Vancomycin resistant Enterococci (VRE)
- Extended spectrum β-lactamase (ESBLs) producing Gram-negative bacteria
- Klebsiella pneumoniae carbapenemase (KPC) producing Gram-negatives
- Imipenem resistant Acinetobacter baumannii
- Imipenem resistant Pseudomonas aerginosa
- Multidrug resistant Mycobacterium tuberculosis (MDR-TB) and extremely drug resistant Mycobacterium tuberculosis (XDR-TB)
Did you Know?
(1) Pittet, Didier; „Adapt to adopt; TEDxPlaceDesNations“, under https://www.youtube.com/watch?v=5tgH0uTqqcE (accessed at 2 May 2016)
(2) WHO; Presentation: WHO_Facts_DRT661; „Health-Care Associated Infection and Hand Hygiene Improvement - Slides for the Hand Hygiene Co-ordinator of the WHO“ http://www.who.int/gpsc/country_work/gpsc_ccisc_fact_sheet_en.pdf
Causes
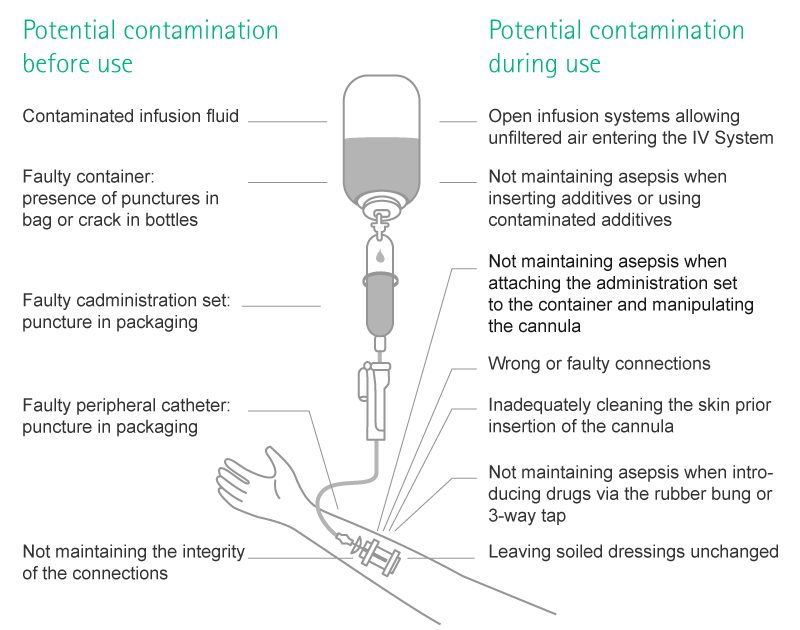
Contamination may occur if pathogens are carried unintendedly from a source to an orifice or an artificial body opening of the host where they then start growing and exerting their harm.
Possible Sources, Entry Routes and Ways for Transmission
There are several possible sources, entry routes and ways for transmission.
- Sources: Natural body orifices or artificial openings due to injury or disease
- Entry portals: Natural body orifices or artificial openings due to injury or disease
- Direct transmission via contact or droplet spread
- Indirect transmission via surfaces or instruments
- Indirect transmission via vectors, mosquitoes, flies, rats transmitting the infection
- Indirect transmission via intermediate host [e.g. human, animal or insect, e.g. transmission of malaria through mosquitoes].
In a health care setting, important ways of contamination are hands of health care personnel and via droplets in the air.
Infusion-Related Infections
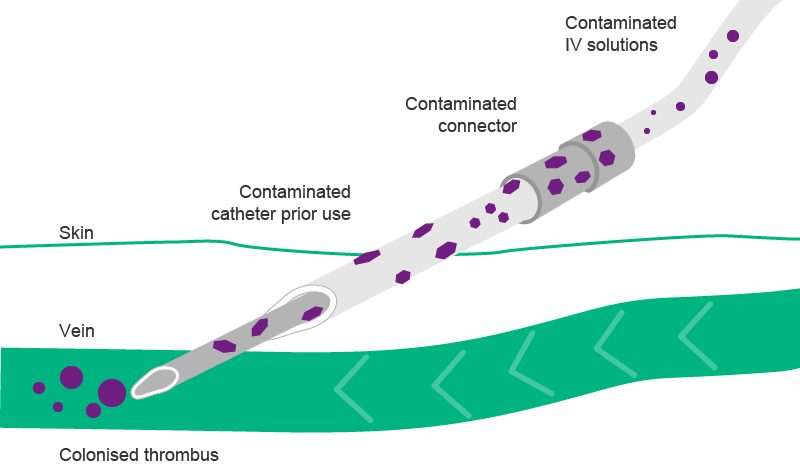
Contamination in infusion settings may occur, when pathogens are carried inside of the infusion system, mostly happening during manipulation.
With regards to infusion-related infections, there are two separate routes: the extra- and the intraluminal route.11,12,13 Intraluminal contamination is the consequence of improper handling of the infusion system, e.g. of the catheter hub at the time of connection and disconnection of the administration set. It is the most common origin of catheter infections after the first week of catheter placement.11,12,13
Health Consequences
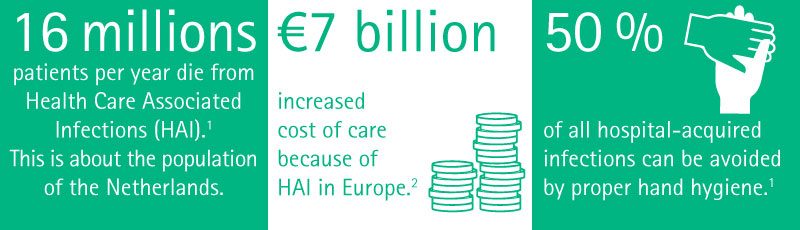
Nosocomial infections occur worldwide and affect both developed and resource-poor countries. Infections acquired in health care settings are among the major causes of death and increased morbidity among hospitalized patients. They are a significant burden both for the patient and for public health. A prevalence survey conducted under the auspices of WHO in 55 hospitals of 14 countries representing 4 WHO Regions (Europe, Eastern Mediterranean, South-East Asia and Western Pacific) showed an average of 8.7% of hospital patients had nosocomial infections. At any time, over 1.4 million people worldwide suffer from infectious complications acquired in hospital.15
These infections occur worldwide both in developed and developing countries. Nosocomial infections accounts for 7% in developed and 10% in developing countries. As these infections occur during hospital stay, they cause prolonged stay, disability, and economic burden.16
The most frequent nosocomial infections are infections of surgical wounds, urinary tract infections and lower respiratory tract infections.
The WHO study, and others, have also shown that the highest prevalence of nosocomial infections occurs in intensive care units and in acute surgical and orthopaedic wards. Infection rates are higher among patients with increased susceptibility because of old age, underlying disease, or chemotherapy.3
Contamination and subsequent infection can occur locally or systematically.
- In case of a local infection, surgical wound infections, skin irritations and catheter entry site infections may occur.
- In case of a systematic inflammation with pathogens reaching the systemic circulation, septicemia, sepsis and septic shock may be the result, as well as pathogens might be transported to organs or extremities and cause organ infection and failure as well as endocarditis or osteomyelitis which might possible result in amputation.17,18
In all cases, additional diagnostic investigation and treatment will be required for a patient, leading to discomfort, emotional stress, pain and potential side effects. In some cases, they might even lead to disabling conditions that reduce the quality of life.
Along with this, the hospital stay might be prolonged. One study19 showed that the overall increase in the duration of hospitalization for patients with surgical wound infections was 8.2 days, ranging from 3 days for gynaecology to 9.9 days for general surgery and 19.8 days for orthopaedic surgery.
The EPIC II point-prevalence study of infection in critically ill patients performed on 8th May 2007 assessed the role of methicillin resistance in survival of patients with Staphylococcus aureus infection. On the study day, 7,087 (51 %) of the 13,796 patients were classified as infected. There were 494 patients with MRSA infections and 505 patients with MSSA (Methicillin-susceptible Staphylococcus aureus) infections. ICU mortality rates were 29.1 % and 20.5 %, respectively (P<0.01) and corresponding hospital mortality rates were 36.4 % and 27.0 % (P<0.01). Multivariate analysis of hospital mortality for MRSA infection showed an adjusted OR (Odds Ratio) of 1.46 (95 % CI 1.03-2.06) (P=0.03).
In ICU patients, MRSA infection is therefore independently associated with an almost 50 % higher likelihood of hospital death compared with MSSA infection.20 Others have found the mortality rate for blood-stream-infections to be 10-25 %, that of septic shock was even higher with 40-60 %.21 Thus, nosocomial infections are one of the leading causes of death.22
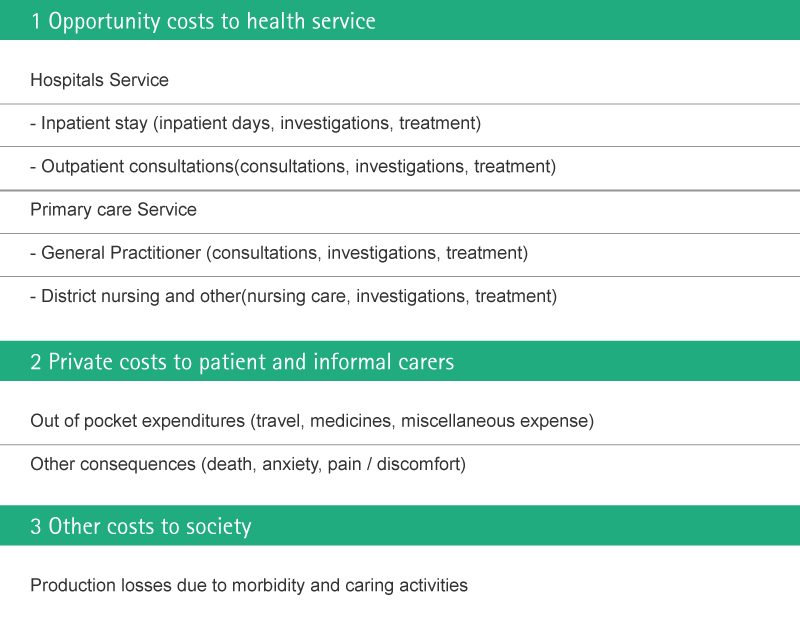
Financial Consequences
Prevention of contamination of medical devices and infusion solutions and thus prevention of severe infection and sepsis is of paramount importance in the hospital setting and can result in tangible savings for the health care provider. In the case of severe sepsis, which requires full ICU treatment, a hospital may save up to € 56,670 per single case.
Economical Impact of nosocomial infections
Uslusoy et al.17 have estimated more than two million cases of nosocomial infections every year (5.7 infections per 100 admissions) with an average cost of € 13,973. They state that in case of MRSA this can be up to € 35,367.
Nosocomial infections occur in more than two million hospitalizations each year.38 The economic costs of nosocomial infections are considerable.37,39 The increased length of stay for infected patients is the greatest contributor to cost.6,19,40 Additionally, increased morbidity and increased total cost per patient who survived is approximately € 40,000.40,41
In their study evaluating the outcome of intravenous catheter related infections in critically ill patients, Rello et al. found that among the survivors, the hospital stay was increased by 19.6 days.42 This added cost of € 3,124 per episode of catheter related infection based on the additional days only, not taking diagnostic and treatment expenses into account.
Vandijck et al. investigated the daily cost of antimicrobial therapy in patients with ICU-aquired bloodstream infection.43 The mean overall daily antimicrobial cost was € 114.25 per patient. As the average duration of antimicrobial therapy for infected patients ranges from 7 to 14 days, the total cost of antimicrobial therapy per patient ranged between € 800 & € 1,200. In special cases of infections from bacteria resistant to comon antibiotics, it has been identified a potential extra cost of $ 8,480/patient (approx. € 5,000).44
A systematic literature review covering 1990-2000 calculated the following average attributable costs (costs calculated with a control group of patients and including only costs directly resultant from nosocomial infection) to the hospital for nosocomial infections:
- Average nosocomial infection, mean cost = $ 13,973
- Bloodstream infection, mean cost = $ 36,441
- Methicillin resistant Staphylococcus aureus infections (MSRA), mean cost = $ 35,367
- Surgical site infection, mean cost = $ 25,546
- Pneumonia, mean cost = $ 9,969.
For the following infections, no studies were done to determine attributable costs but treatment costs are known:
- Urinary tract infection, mean cost = $ 1,008
- Varicella zoster virus, mean cost = $ 27,377
- Tuberculosis, mean cost = $ 61,446
- Measles, mean cost = $ 41,087.
Since the literature review, Roberts et al. created an economic model based upon a sample of patients at Rush University Hospital that controlled for severity of illness and intensive care unit to calculate the average attributable cost of an average nosocomial infection at $ 15,275.41,45
Another recent study utilized national data and a case-control matching method to control DRG, sex, race, age, and comorbidity to calculate that the average excess costs attributable to the national indicator “selected infection due to medical care” are $ 38,656.42
The costs of a nosocomial infection outbreak can easily reach millions of dollars.46
Prolonged stay not only increases direct costs to patients or payers but also indirect costs due to lost work. The need for isolation and the use of additional laboratory and other diagnostic studies also contributes to the costs.
Hospital-acquired infections add to the imbalance between resource allocation for primary and secondary health care by diverting scarce funds to the management of potentially preventable conditions.
On October 1st 2008, the Centers for Medicare and Medicaid Services (CMS) decided to cease paying hospitals for some of the care made necessary by “preventable complications” — conditions that result from medical errors or improper care and that can reasonably be expected to be averted.47
Even non-fatal episodes of microbiological contamination lead to additional involvement for diagnostic (e.g. blood cultures, laboratory work, X-ray) and therapeutic interventions (e.g. antibiotics, catecholamines) as well as an increased length of stay and the average daily cost 48,49,50 of the expected clinical treatment.
Potential Risk Associated Costs
Patients with severe infections and sepsis are generally treated in intensive care units (ICUs). Because of the high proportion of fixed costs in ICU treatment, the total cost of ICU care is mainly dependent on the length of ICU stay (ICU-LOS). The average total cost per ICU day is estimated at approximately € 1,200 for countries with a highly developed healthcare system (based on various studies conducted between 1989 and 2001 and converted at 2003 currency rates).
Those patients require a prolonged ICU-LOS, resulting in higher costs of treatment compared with other ICU patients. US cost-of-illness studies focusing on direct costs per sepsis patient have yielded estimates of € 34,000, whereas European studies have given lower cost estimates, ranging from € 23,000 to € 29,000.Direct costs, however, make up only about 20–30 % of the cost of illness of severe sepsis. Indirect costs associated with severe sepsis account for 70–80 % of costs and arise mainly from productivity losses due to mortality.51
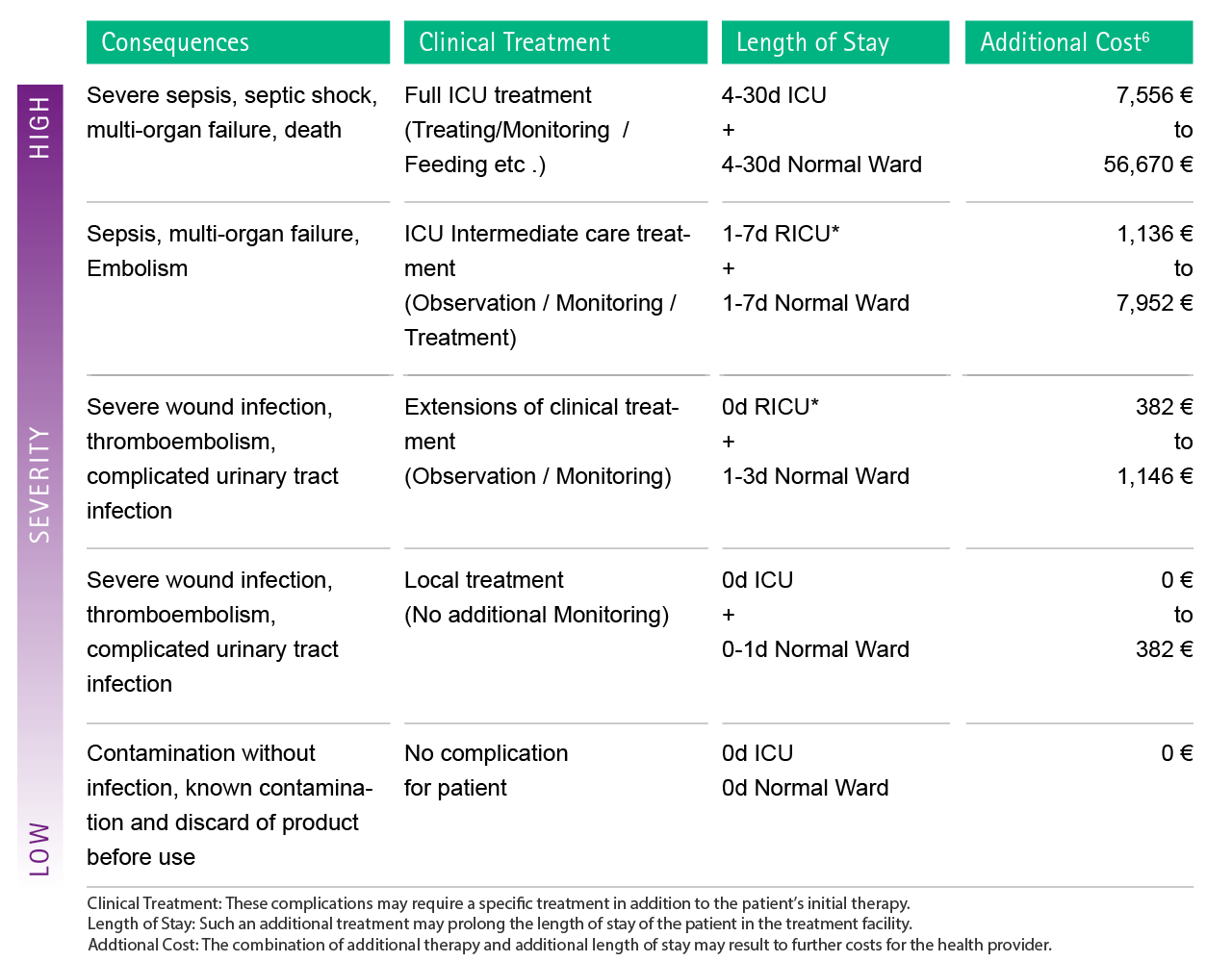
Fig. 4: Estimation of possible additional costs as a consequence of complications caused by microbiological contamination. In order to facilitate the attribution of each complication to the cost calculation, severity levels were introduced. RICU: Respiratory intermediate care unit
The table below gives a schematic representation of the costs associated with Nosocomial Infections.52
Preventive Strategies
Prevention of microbiological contamination and thus nosocomial infection has gained increasing importance and attention throughout the last years because of the dramatic consequences for health and economy.
Medical societies, hospitals and government agencies have invested in development of evidence based guidelines for prevention of nosocomial infections.3,6,9,10,23,24,25
Education and training
Proper education of health care workers with requisite knowledge, skills and attitudes for good infection control practices is the most important measure for infection prevention. Awareness programs, in-service education and on-the-job training with periodic re-training or orientation of staff should be provided. 9,25
Among all measures, hand hygiene has the biggest impact in infection prevention and gloves as well as other personal safety equipment should always be used (see Figure 8, 9). The WHO and CDC have launched a campaign named “Wash your hands”, along with posters, trainings, websites and guidelines on hand hygiene.9,26,27,28 Proper hand hygiene cuts MRSA rates by 50 %.29
Monitoring and surveillance
The implementation of surveillance systems on ICUs and for other patient populations at risk to determine infusion related complication rates, monitor trends and correct lapses in infection control practice have been proven to be successful. E.g. the infection surveillance strategy in the Netherlands were able to reduce MRSA prevalence below 1 % of all clinical isolates and is thus one of the lowest worldwide. 30,31,32
Engineerial and technical solutions
- Use of sterile disposables
- Use of closed systems and devices, that do not exchange unfiltered air or contaminants with the adjacent environment.35
- Use of transparent dressings to secure the cannula / catheter
- Commercially available intravascular solutions are manufactured and provided sterile. Contamination of infusate solutions rarely occurs during the manufacturing process28, but is more likely during manipulation and contamination in course of manual preparation6,9,33,34
Handling
- All intravenous solution containers must be carefully inspected for cracks, defects, turbidity, and particulate matter before preparation and use.
- Intravenous catheters should never be re-inserted.
- Routine replacement of IV administration set according to CDC or local hospital/ institutional guidelines.25
- As less manipulations as possible should be done on infusion systems as every manipulation bears the risk of contamination.
- Maximum sterile barriers should be used whenever possible.
Highlight Safety Products
Scientific Evidence
1 Ghiglione JF, Martin-Laurent F, Pesce S. (2015) Microbial ecotoxicology: an emerging discipline facing contemporary environmental threats. Environ Sci Pollut Res; DOI 10.1007/s11356-015-5763-1
2 Gabriel J. (2008) Infusion therapy. Part two: Prevention and management of complications. Nurs Stand; 22(32): 41-8
3 World Health Organization 2002
4 Ducel G. Les nouveaux risques infectieux. Futuribles. 1995;203:5–32
5 Guembe M, Martín-Rabadán P, Echenagusia A, Camúñez F, Rodríguez-Rosales G, Simó G, Echenagusia M, Bouza E. (2012) How should long-term tunneled central venous catheters be managed in microbiology laboratories in order to provide an accurate diagnosis of colonization? J Clin Microbiol;50(3):1003-7
6 O‘Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML, Raad II, Randolph A, Weinstein RA. 2002
7 European Centre for Disease Prevention and Control. Prevalence of MRSA in Europe 2008
8 Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK; National Healthcare Safety Network Team; Participating National Healthcare Safety Network Facilities. (2008) NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008 Nov;29(11):996-1011
9 Centers for Disease Control and Prevention (CDC)
10 Hebert C, Weber SG. (2011) Common approaches to the control of multidrug-resistant organisms other than methicillin-resistant Staphylococcus aureus (MRSA). Infect Dis Clin North Am. 2011 Mar;25(1):181-200
11 Shah H, Bosch W, Thompson KM, Hellinger WC. (2013) Intravascular catheter-related bloodstream infection. Neurohospitalist; 3(3): 144-51
12 Mermel LA. (2011) What is the predominant source of intravascular catheter infections? Clin Infect Dis. 2011 Jan 15;52(2):211-2
13 Rosado V, Romanelli RM, Camargos PA. (2011) Risk factors and preventive measures for catheter-related bloodstream infections. J Pediatr (Rio J); 87(6): 469-77
14 Jamieson EM, McCall JM, Whyte LA. Practice 21: Intravenous therapy. In: Jamieson EM, McCall JM, Whyte LA. Clinical nursing practices. 5. Edition, Edinburgh [u.a.]: Elsevier Churchill Livingstone 2007; 169-176
15 WHO, 2002, Prevention of hospital-acquired infections. A practical guide. 2nd edition
16 Khan, Hassan Ahmed; Baig, Fatima Kanwal; Mehboob, Riffat (2017): Nosocomial infections. Epidemiology, prevention, control and surveillance. In Asian Pacific Journal of Tropical Biomedicine 7 (5), pp. 478–482. DOI: 10.1016/j.apjtb.2017.01.019
17 Uslusoy E., Mete S. (2008) Predisposing factors to phlebitis in patients with peripheral intravenous catheters: a descriptive study. J Am Acad Nurse Pract; 20(4): 172-80
18 Bouchoucha S, Benghachame F, Trifa M, Saied W, Douira W, Nessib MN, Ghachem MB. (2010) Deep venous thrombosis associated with acute hematogenous osteomyelitis in children. Orthop Traumatol Surg Res; 96(8): 890-3
19 Raad I. (1998) Intravascular-catheter-related infections.Lancet; 351(9106): 893-8.
20 Hanberger H, Walther S, Leone M, Barie PS, Rello J, Lipman J, Marshall JC, Anzueto A, Sakr Y, Pickkers P, Felleiter P, Engoren M, Vincent JL; EPIC II Group of Investigators. (2011) Increased mortality associated with methicillin-resistant Staphylococcus aureus (MRSA) infection in the intensive care unit: results from the EPIC II study. Int J Antimicrob Agents; 38(4): 331-5
21 Rosenthal VD, Maki DG. (2004) Prospective study of the impact of open and closed infusion systems on rates of central venous catheter-associated bacteremia. Am J Infect Control; 32(3): 135-41.
22 Gastmeier P, Geffers C, Brandt C, Zuschneid I, Sohr D, Schwab F, Behnke M, Daschner F, Rüden H. (2006) Effectiveness of a nationwide nosocomial infection surveillance system for reducing nosocomial infections. J Hosp Infect; 64(1): 16-22
23 Zingg W, Holmes A, Dettenkofer M, Goetting T, Secci F, Clack L, Allegranzi B, Magiorakos AP, Pittet D; systematic review and evidence-based guidance on organization of hospital infection control programmes (SIGHT) study group. (2015) Hospital organisation, management, and structure for prevention of health-care-associated infection: a systematic review and expert consensus. Lancet Infect Dis. 2015; 15(2): 212-24
24 Sax H, Clack L, Touveneau S, Jantarada Fda L, Pittet D, Zingg W; PROHIBIT study group. (2013) Implementation of infection control best practice in intensive care units throughout Europe: a mixed-method evaluation study. Implement Sci; 8: 24
25 World Health Organization. 2004
26 Royal College of Nursing (RCN). 2010
27 World Health Organization. 2009 World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. WHO Library Cataloguing-in-Publication Data,
28 MMWR Morbitity and Mortality Weekly Report. 2002 Morbitity and Mortality Weekly Report. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Recommendations and Reports, Oct 25, 2002, (51) No. RR-16
29 Grayson ML, Jarvie LJ, Martin R, Johnson PD, Jodoin ME, McMullan C, Gregory RH, Bellis K, Cunnington K, Wilson FL, Quin D, Kelly AM, 2008
33 Royal College of Nursing. 2005
34 Scales K. Vascular access: a guide to peripheral venous cannulation. Nurs Stand. 2005; 19(49): 48-52
35 NIOSH. Preventing Occupational Exposures to Antineoplastic and other Hazardous Drugs in Healthcare Settings. 2004
bloodstream infection. International Journal of Antimicrobial Agents 2008; 31: 161-165
52 Plowman RP, Graves N, Robers JA. Hospital Aquired Infection. Office of Health Economics, London 1997