Particulate Contamination
Particulate contamination describes the unintended presence of extraneous, mobile and undissolved particles in a parenteral solution.1,2 These particles can be of various size, defining them as detectable by visual inspection (in general ≥ 50 µm) sub-visible inspection with a range of 2-50 µm in size in general. Especially the sub-visible sized particles demand specific analytical tests for their detection.
Did you Know?
(1) Ortolani GA, Russell RL, Angelbeck JA, Schaffer J, Wenz B. (2004) Contamination control in nursing with filtration. Part 1: filters applied to intravenous fluids and point-of-use hospital water. J Infus Nurs; 27(2): 89-103
(2) Anonymus [No authors listed]. (2004) Risks due to particles in infusion therapy--experts promote use of infusion filters. Krankenpfl J. 2004; 42(3-4): 97
(3) Lehr HA, Brunner J, Rangoonwala R, Kirkpatrick CJ. (2002) Particulate matter contamination of intravenous antibiotics aggravates loss of functional capillary density in postischemic striated muscle. Am J Respir Crit Care Med; 165:514-520
Causes
Several causes of particulate contaminations of IV fluids are known. This is because drugs are available in various containers (for example vials, ampoules, pre-filled containers and premixed solutions) and their usage and manipulations are highly diversified. Consequently, many types of particulate contamination can occur:
Glass
Glass ampoules especially pose a high risk of particulate contamination as glass fragments may enter the ampoule when it is opened.3 If a needle (for example 18G) is used for removing a glass ampoule’s content, small glass particles can pass through the needle into the syringe and easily be injected into patients.
This risk remains if drugs are routinely administered via the injection port of the intravenous cannula, which is a safety measure designed to decrease sharp injuries to the medical staff.3,4
Plastic contamination
Plastic contamination occurs frequently due to:
- particles from the infusion container’s raw material itself
- the injection port due to its usage with sharp items
Rubber
The insertion of a needle through the stopper of a medication vial or infusion container can shear off a small piece of the stopper. This particle may float in the medication or IV solution. If the particle is small or masked (e.g. by the label, a matching background or a colored vial), the contamination may be unnoticed. The particle may then be aspirated into a syringe and injected into a patient.5
Undissolved solids
Undissolved solids in drugs or parenteral solutions can also be an origin of particulate contamination.6,7
Health Consequences
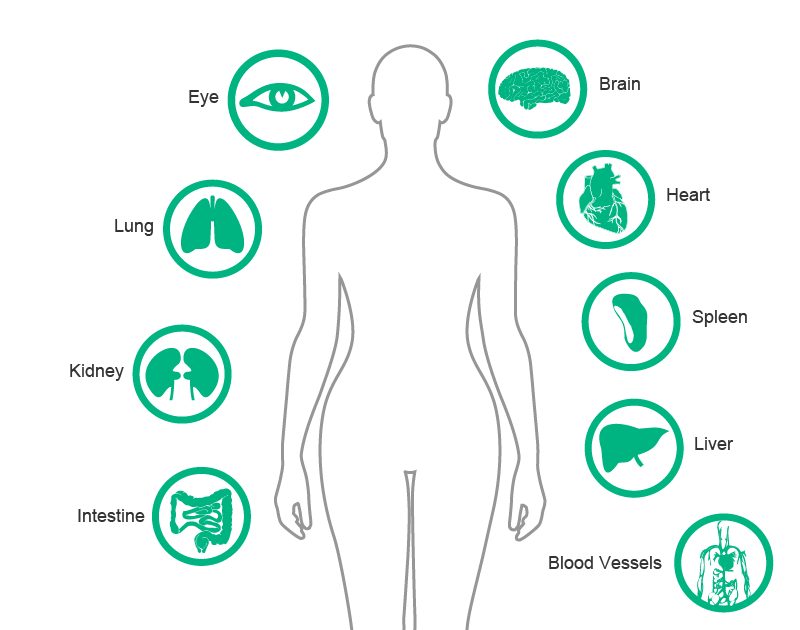
All types of particles present in the intraluminal compartment, which are not eliminated by a filter, are directly entering the human body. These particles from plastic, glass or rubber can have unfavorable effects, especially in patients who are already ill.
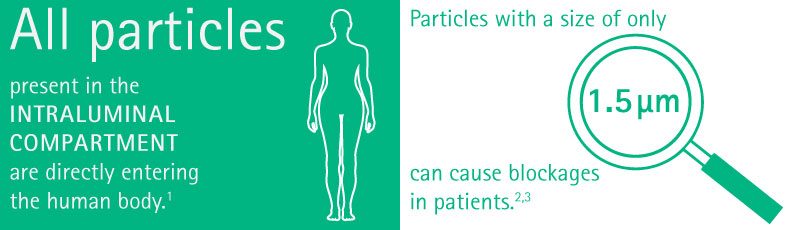
Particles as small as 1.5 µm can cause blockages in patients, whereas particles of 6 µm can cause blockages in healthy subjects.7,8,11
Damage to various organs, such as the lungs, kidneys, liver and spleen are described in general1,6 but particularly affected are severely ill patients.8-11 Patients with prior organ damage are especially sensitive, as particles can exacerbate their impaired blood micro-circulation.8,11
A further clinical sign that can be caused by glass particles from glass ampoules is phlebitis.7,8,12 Phlebitis is evident as local warmth, with pain, swelling and reddening at the affected site of parenteral administration.13
Financial Consequences
Given the wide spectrum of patient complications caused by the various particles from plastic, glass and rubber found as contamination, it has to be assumed that particulate contamination can lead or contribute to extended duration of hospital stay as well as additional treatment costs.
Potential Risk Associated Cost
A cost evaluation of the risk can be done by assigning costs to their related clinical treatment and resulting extended length of stay. The cost can be calculated using the average daily cost of the expected clinical treatment.
The Fig. 2 below shows the results of such a calculation for selected examples of complications.
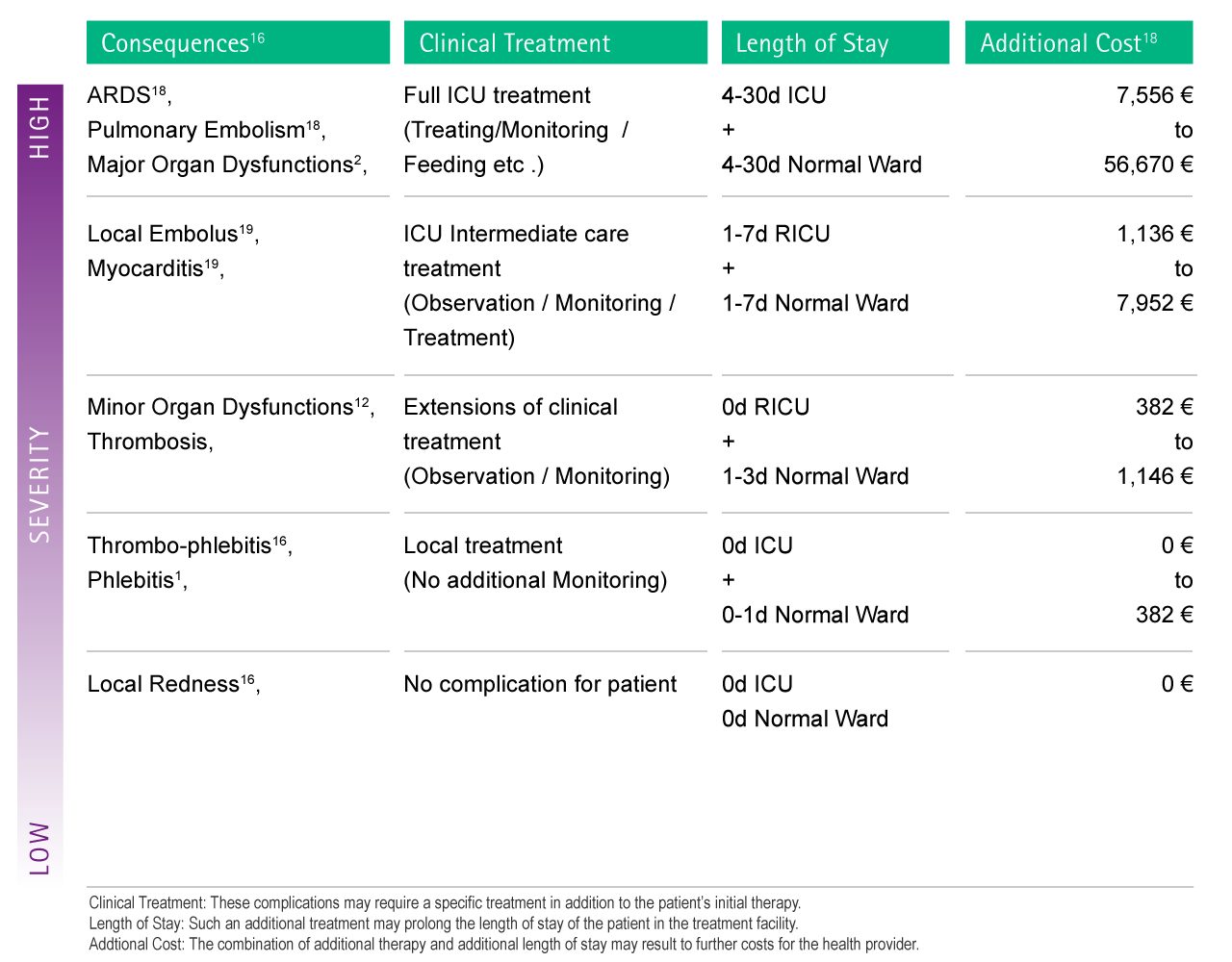
According to the severity of its direct and indirect complications, the entrance of foreign particles into the patient's circulatory system may lead to an additional cost for the healthcare provider of up to 56,670 € per single case.16-19
Preventive Strategies
The strategy to prevent particle contamination at the point of origin is based on many aspects:
- using quality products to avoid the generation of particles (e.g. stopper of vials)
- using products with a low intrinsic particle load (e.g. plastic containers instead of glass ampoules)
- avoid drug incompatibilities
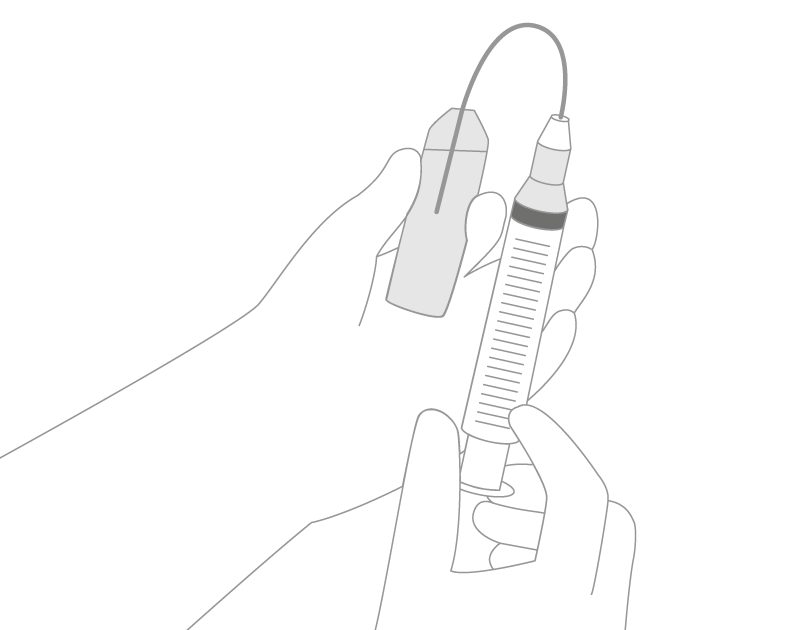
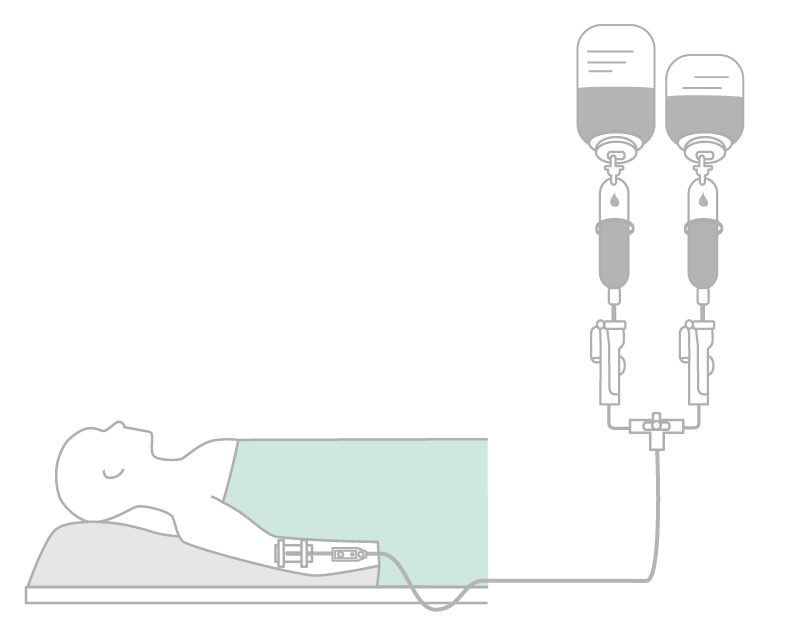
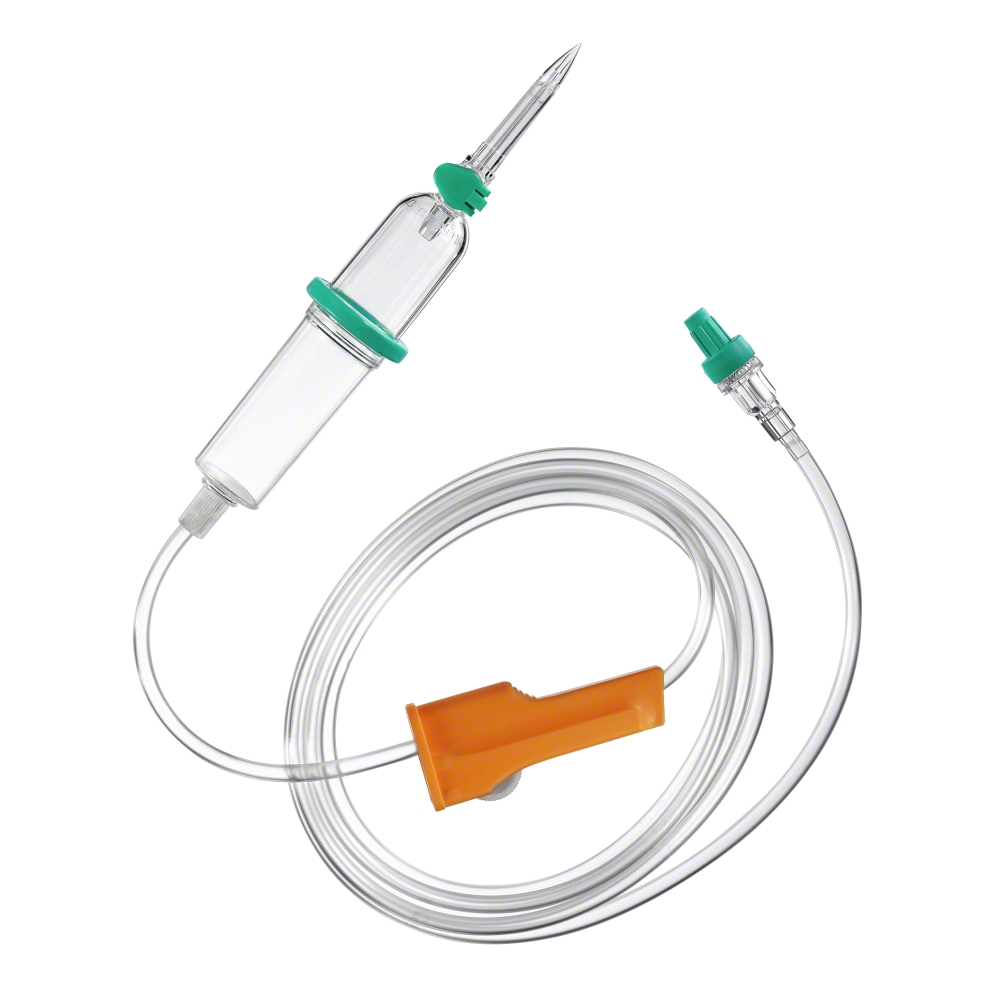
Particle contamination may be reduced by using an in-line filter and filter needle to withdraw drugs from glass ampoules prior to administration. The in-line filter devices can remove:
- particulate contamination
- microbiological contamination
- air from infusion solutions.
If a particulate contamination has occurred, the usage of in-line filters provides an important safety benefit. In addition, filters function:
- as an early warning system
- providing optical control of the infusate
- stopping the infusion when the filter gets obstructed.
It is, however, not standard practice to perform filtration. Filters in the intravenous line may be positioned close to the patient access.
In-line filters are recommended for:
- non lipid-containing solutions (0.2 μm filter)14
- lipid infusions or total nutrient preparations (1.2 μm filter)14
- the ISO 8536-4 norm (for infusion sets) recommends filtration for patient protection. (Generally, the fluid filter used has a nominal pore size of 15 µm [ISO 8536-4].
The British Pharmaceutical Nutrition Group (BPNG) has issued a guideline about how to avoid contaminating the body with insoluble particles.14,15
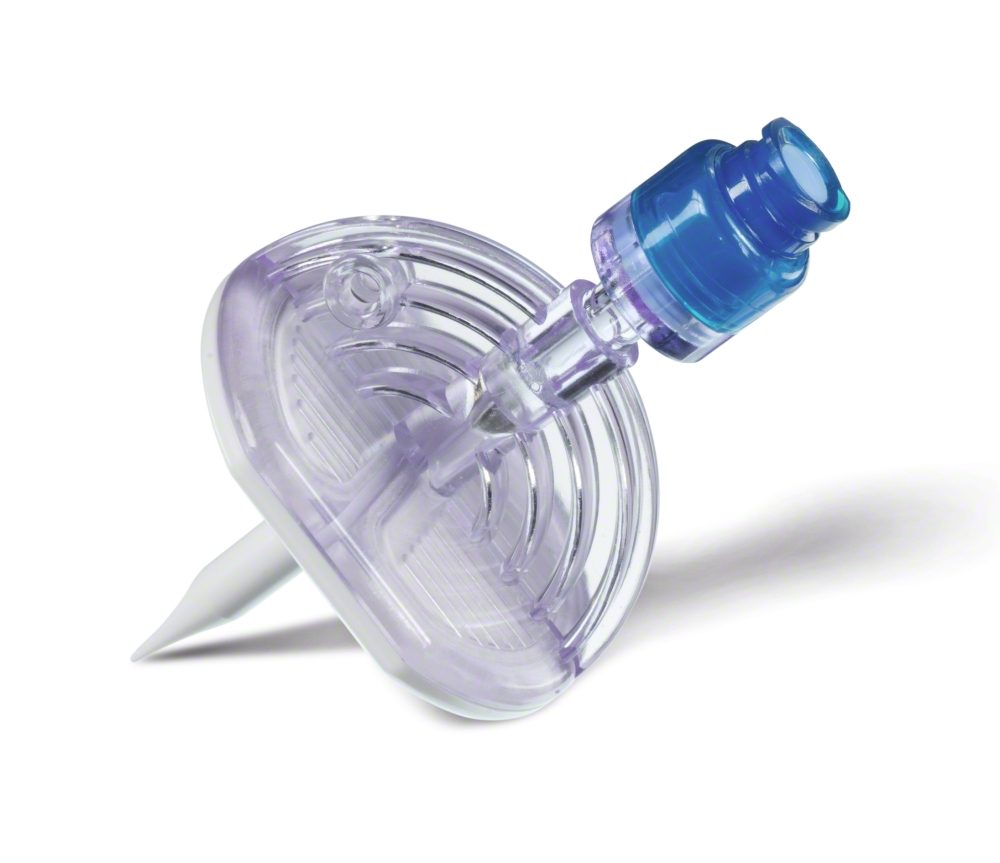
- solutions being added to parenteral nutrition taken from glass ampoules or vials should be added to the final parenteral nutrition admixture through a filter with a maximum pore size of 5 μm. Filter needles and dispensing pins (spikes) with particle filter minimize the risk of injecting glass particles into patients (Fig. 3).
- appropriate filters should be used during the administration of parenteral nutrition to patients who require intensive or prolonged parenteral therapy, namely immunocompromised patients, neonates and children, and patients receiving home parenteral nutrition.
- the 1.2 μm filters should be used for the administration of solutions containing lipids, including all-in-one admixtures, and changed every 24 hours.
Highlight Safety Products
Scientific Evidence
1 Werner BP, Winter G. (2015) Particle contamination of parenteralia and in-line filtration of proteinaceous drugs. Int J Pharm;496(2):250-67
2 Doessegger L, Mahler HC, Szczesny P, Rockstroh H, Kallmeyer G, Langenkamp A, Herrmann J, Famulare J. (2012) The potential clinical relevance of visible particles in parenteral drugs. J Pharm Sci; 101(8): 2635-44
3 Lee KR, Chae YJ, Cho SE, Chung SJ. (2011) A strategy for reducing particulate contamination on opening glass ampoules and development of evaluation methods for its application. Drug Dev Ind Pharm; 37(12): 1394-401
4 Lye ST, Hwang NC. (2003) Glass particle contamination: is it here to stay? Anaesthesia; 58(1): 93-4
5 Roth JV. (2007) How to enter a medication vial without coring. Anesth Analg; 104(6): 1615
6 Boehne M, Jack T, Köditz H, Seidemann K, Schmidt F, Abura M, Bertram H, Sasse M. (2013) In-line filtration minimizes organ dysfunction: new aspects from a prospective, randomized, controlled trial. BMC Pediatr; 13 : 21
7 Ortolani GA, Russell RL, Angelbeck JA, Schaffer J, Wenz B. (2004) Contamination control in nursing with filtration. Part 1: filters applied to intravenous fluids and point-of-use hospital water. J Infus Nurs; 27(2): 89-103
8 Anonymus [No authors listed]. (2004) Risks due to particles in infusion therapy--experts promote use of infusion filters. Krankenpfl J. 2004; 42(3-4): 97
9 Jack T, Boehne M, Brent BE, Hoy L, Köditz H, Wessel A, Sasse M. (2012) In-line filtration reduces severe complications and length of stay on pediatric intensive care unit: a prospective, randomized, controlled trial. Intensive Care Med; 38(6): 1008-1
10 Oie S, Kamiya A. (2005) Particulate and microbial contamination in in-use admixed parenteral nutrition solutions. Biol Pharm Bull; 28(12): 2268-70
11 Lehr HA, Brunner J, Rangoonwala R, Kirkpatrick CJ. (2002) Particulate matter contamination of intravenous antibiotics aggravates loss of functional capillary density in postischemic striated muscle. Am J Respir Crit Care Med; 165:514-520
http://www.ncbi.nlm.nih.gov/pubmed/11850345
12 Yorioka K, Oie S, Oomaki M, Imamura A, Kamiya A. (2006) Particulate and microbial contamination in in-use admixed intravenous infusions. Biol Pharm Bull;29(11):2321-3
http://www.ncbi.nlm.nih.gov/pubmed/17077539
13 Nassaji-Zavareh M, Ghorbani R. Peripheral intravenous catheter-related phlebitis and related risk factors. Singapore Med J. 2007 Aug;48(8):733-6
14 Ball PA. (2003) Intravenous in-line filters: filtering the evidence. Curr Opin Clin Nutr Metab Care; 6(3): 319-25
15 Bethune K Allwood M, Grainger C, Wormleighton C; British Pharmaceutical Nutrition Group Working Party. (2001) Use of filters during the preparation and administration of parenteral nutrition: position paper and guidelines prepared by a British pharmaceutical nutrition group working party. Nutrition;17(5):403-8
16 Gianino MM, Vallino A, Minniti D, Abbona F, Mineccia C, Silvaplana P and Zotti CM. A method to determine hospital costs associated with nosocomial infections (transl). Ann Ig 2007; 19(4): 381-92
http://www.ncbi.nlm.nih.gov/pubmed/17937330
17 Kossovsky N, Cole P, Zackson DA. Giant cell myocarditis associated with silicone. An unusual case of biomaterials pathology discovered at autopsy using X-ray energy spectroscopic techniques. Am J Clin Pathol 1990; 93(1): 148-52
http://www.ncbi.nlm.nih.gov/pubmed/2294695
18 Walpot H, Franke RP, Burchard WG, Agtermkamp C, Müller FG, Mittermayer C, Kalff G. The filter effectiveness of common 15-micron filters (DIN 58362). II: Scanning electron microscopy and roentgen analysis. Infusionstherapie 1989; 16(3): 133-9
http://www.ncbi.nlm.nih.gov/pubmed/2503453
19 Preston ST, Hegadoren K. Glass contamination in parenterally administered medication. J Adv Nurs 2004; 48(3): 266-70