Drug Incompatibility
Incompatibility is an undesirable reaction that occurs between the drug and the solution, container or another drug. The two types of incompatibilities associated with intravenous administration are physical and chemical.1,2
A drug interaction describes the alteration of a drug effect due to the influence of another substance (i.e. drug, chemical substance, nutrition) resulting in a solution that is no longer optimal for the patient after the substances are mixed.1,3
Did you Know?

(1) Taxis K, Barber N. (2004) Incidence and severity of intravenous drug errors in a German hospital. Eur J Clin Pharmacol; 59(11): 815-7
(2) Emami S, Hamishehkar H, Mahmoodpoor A, Mashayekhi S, Asgharian P. (2012) Errors of oral medication administration in a patient with enteral feeding tube. J Res Pharm Pract; (1): 37-40
Causes
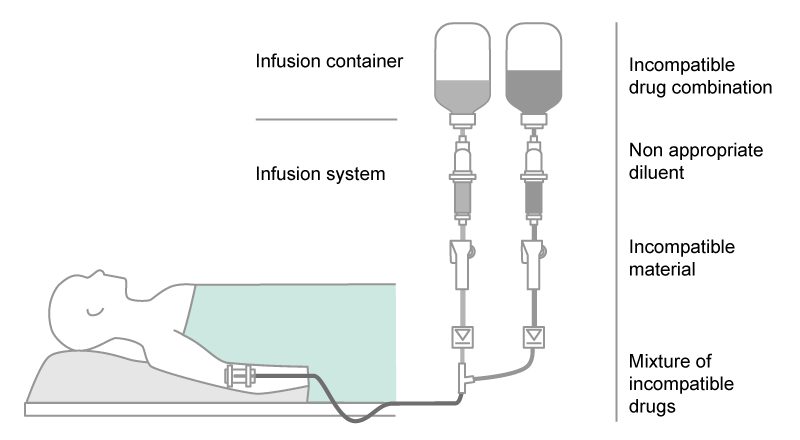
Incompatibilities of drugs in standard IV therapy can occur between:
- drugs and inappropriate IV solutions as diluent
- two drugs (drug-drug incompatibility) when they are
- mixed together, e.g. within the same infusion line (simultaneous infusion) and/or
IV container
- administered one after the other, but within the same infusion line - drugs and adjuvants (preservative, buffer, stabilizer, solvent)
- drugs and materials of IV containers (e.g. PVC) or medical devices, which can concern the nature of the material used and/or reactions at the inner surface (e.g. adsorption).1,3,4,5
Health Consequences
The unintended presence of precipitation and toxic products can cause various negative consequences for the patient.
- damage from toxic products
- particulate emboli from crystallization and separation
- tissue irritation due to major pH changes
- therapeutic failure
The extent of the damage mainly depends on the patient’s condition (age, weight, nature, severity of the disease etc.) and on the type of drug administered. Consequences of physicochemical drug incompatibilities are particularly severe in neonate and pediatric patients.1,4,6
There is little published scientific information about the frequency of drug incompatibility reactions. In one study, incompatibility was investigated in a pediatric intensive care ward showing that 3.4% of drug combinations were incompatible and thus potentially dangerous.7 A life threatening nature was found for 26% of incompatibilities in an intensive care unit (ICU).8
Another survey collected 78 different medication regimes and found 15% with incompatibility reactions.9 Taxis and Barber10 reported that in the ICU clinical incompatibilities can contribute to 25% of medication errors. Further publications showed that, depending on the ward type, up to 80% of IV drug doses were prepared with the wrong diluents.11
Financial Consequences
Adverse effects of drug incompatibilities extend periods of patients’ hospitalization and the total costs for hospitals.
A cost evaluation of the risk can be done by assigning costs to their related clinical treatment and resulting extended length of stay. The cost can be calculated using the average daily cost of the expected clinical treatment.
Potential Risk Associated Cost
The Fig. 2 below shows the results of a calculation for selected examples of complications.
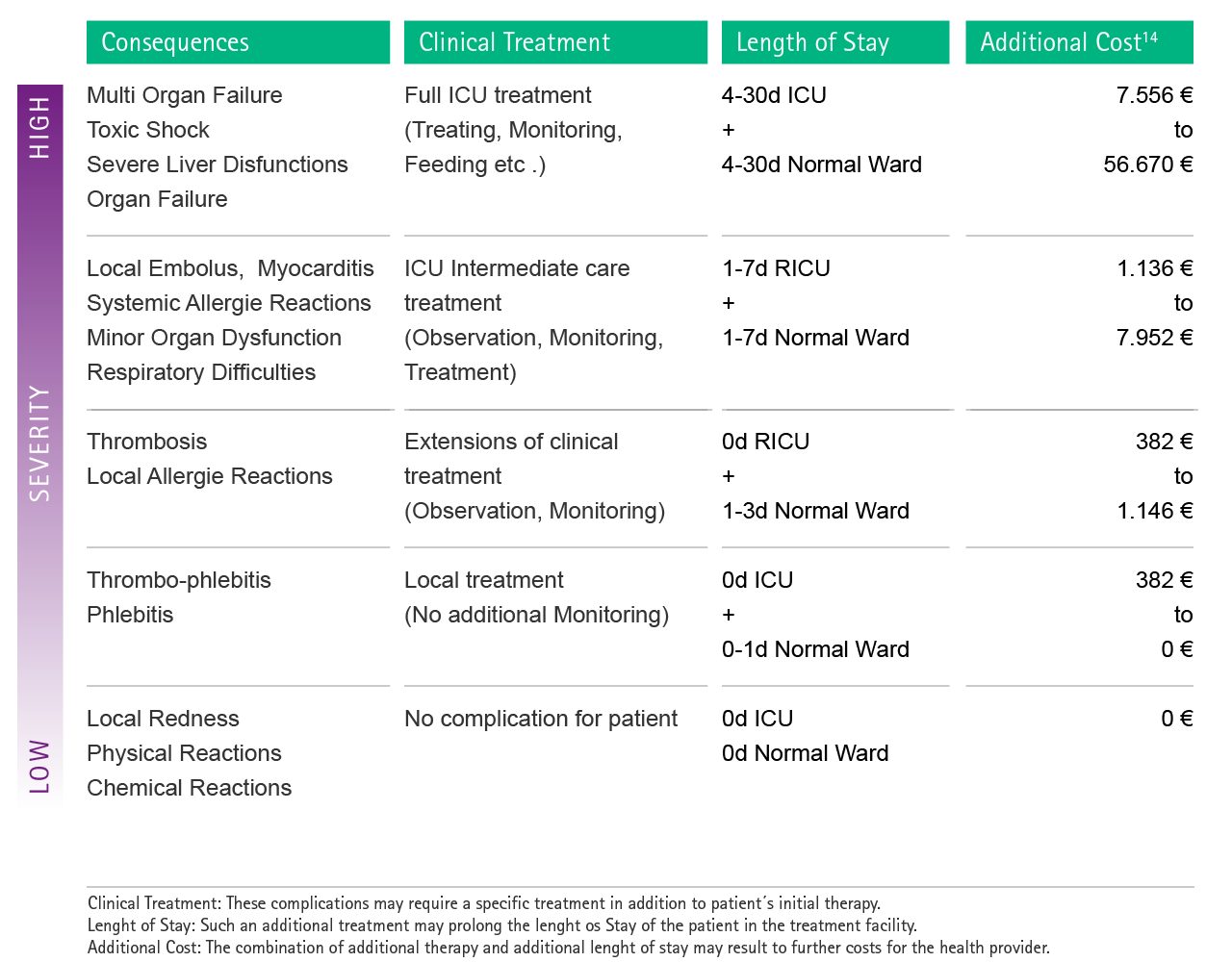
Fig. 2: Estimation of possible additional costs as a consequence of complications caused by drug incompatibilities. In order to facilitate the attribution of each complication to the cost calculation, severity levels were introduced. RICU: Respiratory Intermediate Care Unit
Subsequently, severe respiratory complications caused by toxic drug-drug interactions may lead to an additional cost for the healthcare provider of up to 56,670 € per single case.14
Preventive Strategies
To prevent dangerous incompatibilities and ensure safe patient treatment, it is important to combine various actions in different processes.
- Dangerous incompatibilities can be prevented by a plausibility check regarding the SPC and available sources on compatibility information, also considering the material used for therapy (e.g. diluent, IV container, IV lines) and the infusion regimen.
- Assessment and planning of regimes to avoid mixing of drugs, which have to be administered separately.
- Individual labelling for each drug preparation (including drug, concentration, patient name).
- Separating the drug doses by time and place. This can include the rinsing of the infusion system with a neutral IV solution prior to the application of another drug.2,5,6,9,12
- Consistently checking alternative modes of administration and/or using multi-lumen catheters.
- Use of appropriate in-line filters can reduce influx of particles which result from incompatibilities. In-line filters are able to retain solid particles of at least 0.2 μm.13 As a consequence, the filter may block. This is not a malfunction of the filter, but should initiate a check of the medication in order to eliminate any incompatibility.
Highlight Safety Products
Scientific Evidence
1 Fahimi F, Sefidani Forough A, Taghikhani S, Saliminejad L. (2015) The rate of Physicochemical Incompatibilities, Administration Errors. Factors correlating with Nurses’ errors. Iran J Pharm Res; 14(suppl); 87-93
2 RCN (2010) Royal College of Nursing. Standards for Infusion Therapy.
3 Emami S, Hamishehkar H, Mahmoodpoor A, Mashayekhi S, Asgharian P. (2012) Errors of oral medication administration in a patient with enteral feeding tube. J Res Pharm Pract; (1): 37-40
4 Vijayakumar A, Sharon EV, Teena J, Nobil S, Nazeer I. (2014) A clinical study on drug-related problems associated with intravenous drug administration. J Basic Clin Pharm; 5(2): 49-53
5 Westbrook JI, Rob MI, Woods A, Parry D. (2011) Errors in the administration of intravenous medications in hospital and the role of correct procedures and nurse experience. BMJ Qual Saf; 20(12): 1027-34
6 Höpner JH, Schulte A, Thiessen J, Knuf M, Huth RG. (2007) Preparation of a compatibility chart for intravenous drug therapy in neonatal and pediatric intensive care units. Klin Padiatr; 219(1): 37-43
7 Gikic M, Di Paolo ER, Pannatier A, Cotting J. (2000) Evaluation of physicochemical incompatibilities during parenteral drug administration in a paediatric intensive care unit. Pharm World Sci; 22(3): 88-91
8 Tissot E, Cornette C, Demoly P, Jacquet M, Barale F, Capellier G. (1999) Medication errors at the administration stage in an intensive care unit. Intensive Care Med; 25(4): 353-9
9 Vogel Kahmann I, Bürki R, et al. (2003) Incompatibility reactions in the intensive care unit. Five years after the implementation of a simple "colour code system". Anaesthesist; 52(5): 409-12
10 Taxis K, Barber N. (2004) Incidence and severity of intravenous drug errors in a German hospital. Eur J Clin Pharmacol; 59(11): 815-7
11 Cousins, B Sabatier, D Begue, C Schmitt, and T Hoppe-Tichy. (2005) Medication errors in intravenous drug preparation and administration: a multicentre audit in the UK, Germany and France. Qual Saf Health Care; 14(3): 190-195
12 Riemann T, Schröder F. (2005) More effective prevention of incompatibility reactions through the use of four lumen central venous catheters in critically ill patients. PflegenIntensiv; 2(1): 57
13 Jack T, Boehne M, Brent BE, Hoy L, Köditz H, Wessel A, Sasse M. (2012) In-line filtration reduces severe complications and length of stay on pediatric intensive care unit: a prospective, randomized, controlled trial. Intensive Care Med; 38(6):1 008-16
14 Gianino MM, Vallino A, Minniti D, Abbona F, Mineccia C, Silvaplana P and Zotti CM. A method to determine hospital costs associated with nosocomial infections (transl), Ann Ig 2007; 19(4): 381-392 http://www.ncbi.nlm.nih.gov/pubmed/17937330