„It may be part of human nature to err, but it is also part of human nature to create solutions, find better alternatives and meet the challenges ahead.“
What are Medication Errors?
Medication error is defined as any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is under the control of the healthcare professional, patient, or consumer.4 Medication errors can be classified by considering the types of errors occurring, such as wrong patient, dose, infusion rate, delivery route or medication.
Medication errors may occur during any phase of the drug delivery process from prescription to drug administration and at anywhere medications are administered.3 Errors may occur with any medication; however, chemotherapy presents unique dangers due to narrow therapeutic indices, potential toxicity even at therapeutic dosages, complex regimens, and a vulnerable cancer patient population.6
Medication Errors in Chemotherapy
Past studies have suggested that medication errors account for a significant percentage, if not a majority, of both total medical errors and medical misadventures resulting in mortality. Thus, medication errors are an important subgroup of medical errors due to their frequency, possibility of significant patient harm, and potential for prevention.6 A study revealed that antineoplastic agents were the second most common cause of fatal medication errors10 and antineoplastic drugs have been shown to be the drug-class which most commonly associated with medication error, only after anti-infective drugs.7
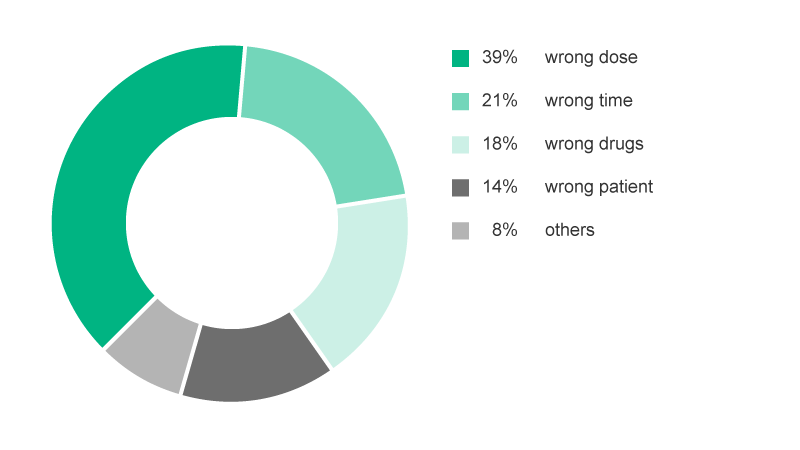
On a general level, of the chemotherapy medication errors reported, 39% involved over- and underdosing, 21% involved schedule and timing errors, 18% involved wrong drugs, and 14% involved chemotherapy given to the wrong patient. Less common errors included infusion-rate errors, omission of drugs or hydration, and improper preparation of drugs. Ten percent of these errors required medical intervention and prolonged hospital stays.8
Cheat Sheets
Risk: Wrong Dose
Generally speaking, wrong drug dosages can occur during all steps of the treatment process. During a 2-year period (2003-2004), Ford6 conducted a prospective study on the oncology ward of a large community hospital on medication errors. They identified 141 errors in total, thereof 38 were classified as “wrong dose”. Of these, one occurred during ordering / transcription, 20 during dispersion and 17 during application. Additional 18 errors were “medication not given”.
Case Study
-
Wrong Dose
In Adelaide (Australia) over a six-month period in 2014 and 2015, 10 cancer patients were given inadequate doses of the drug Cytarabine [10,11]
Prevention of wrong dose errors
Risk: Wrong infusion rate
Marked inaccuracies in IV fluid infusion rates are common, and do not seem to be perceived by staff as important. Metered pumps have been shown to improve accuracy. Rooker et al.13 studied the IV infusion rates in patients requiring continuous IV fluids during a four-week period. The periods over which iv crystalloid fluid bags were administered were compared with the time prescribed.
Checklists for Healthcare Workers
Did you know?
Risk: Wrong delivery route
Most of the chemotherapy regimes are given intravenously, i.e. directly into the venous system. Peripheral venous access may be suitable, however, given the high toxicity of the drugs, mostly central venous access are preferred.
Case Study
-
-
Wrong Delivery Route
A 6-year-old girl received intrathecal medication during her outpatient treatment. 3 days later she presented at the emergency room with pain in her neck and legs ...[14]
Patient Access Routes
Some of the chemotherapy regimen require other patient access routes, such as intra-arterial access for isolated organ perfusion (e.g. for hepatic metastases) or intra-thecal applications (into the spinal canal, through a lumbar injection).
Cytarabine (Ara-C) can be used intrathecally for carcinomatous meningitis due to lymphoma or leukemia, and methotrexate can be given intrathecally for carcinomatous meningitis due to breast and bronchial cancers.15
For example, a patient with highly malignant non-Hodgkin’s lymphoma with cancerous meningitis would typically receive Vincristine 2 mg IV plus Methotrexate 10-15 mg intrathecally.
Carcinomatous meningitis
Some cancers can lead to metastases on the meninges, called carcinomatous meningitis. It occurs often in patients with leukemia or lymphoma, but also in breast cancer and bronchial cancer or malignant melanoma.
Patients with solid tumors plus carcinomatous meningitis have a bad prognosis. Without treatment, their mean survival time is mostly a few weeks only.
Importance of correct delivery route
Vincristine is a vinca alkaloid antineoplastic agent intended for iv use only. Vincristine should never be administered subcutaneously, intramuscularly or intrathecally, as this results in necrosis.14 Accidental vincristine administration via the spinal route (intrathecally via a lumbar puncture or intraventricularly via an Ommaya reservoir) causes rapid sensory and motor dysfunction, usually followed by encephalopathy, coma, and death.16 Thus it has to be made sure that with combination chemotherapy, each drug is given into the correct patient access port.
Route delivery errors
Route delivery errors, of which intrathecal vincristine delivery is one example (there are many other examples such as intravenous delivery of benzathine penicillin, a galenic depot-formulation for intramuscular use), account for 5% of medication errors.17
The specific risks of maladministration of vincristine sulfate were clearly recognized from the early experience in the 1960s.18 However, since then, 58 cases of intrathecal vincristine errors are known to have occurred which have been reviewed intensively.18,14 Out of these cases with inadvertent intrathecal administration of vincristine, only eight patients survived, most of them paralyzed.14 Specific case reports have been published.14,19,20
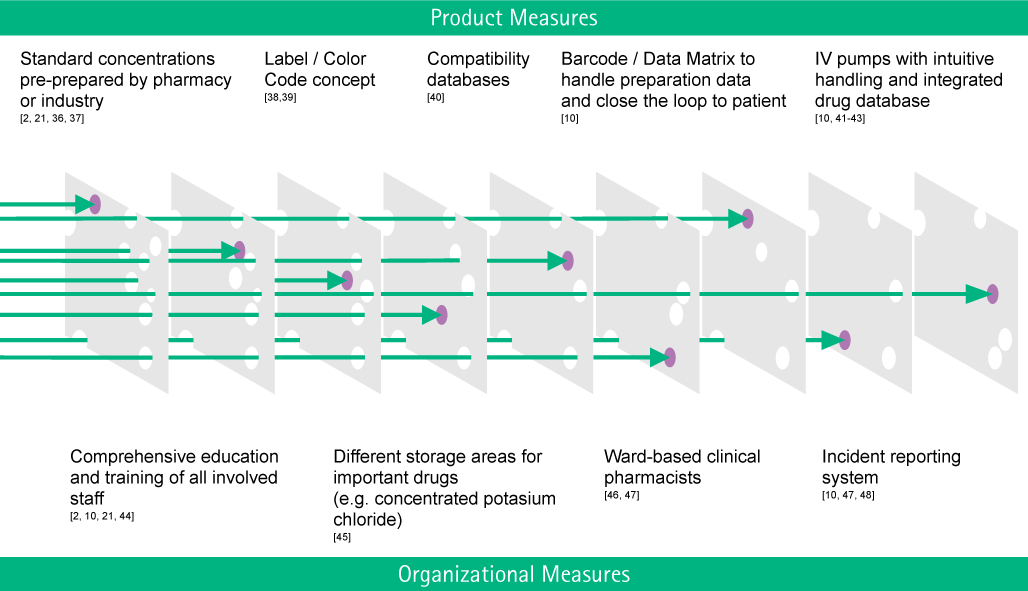
Preventive Strategies
Safeguards used to stop certain drugs being given intrathecally21
Most hospitals have strict rules to prevent the administration of vincristine and other vinca alkaloids into the cerebrospinal fluid. The rules at the Great Ormond Street Hospital for Children, London, for example, state that:
- Cytotoxic drugs should be given only by specialist, appropriately trained staff
- The dose of vinca alkaloid should be diluted to at least 10 ml to help distinguish it from drugs intended for intrathecal injection, for which such a large volume is rarely given
- All administration devices containing vinca alkaloids must be labelled: “Warning: Vin…(drug name): For intravenous use only”
- Intrathecal drugs should be administered in a designated area — for example, an operating theater
- Drugs for intrathecal use should be delivered to the point of use from the pharmacy at a different time and packed separately from other drugs
- No other cytotoxic drugs should be delivered to or stored in such a designated area
Products help preventing wrong delivery route
The universally compatible Luer connector has been identified as a key element in ‘wrong route’ medication errors. Luer connectors are widely used for delivery of infusions. Literature shows that any patient having multiple access systems in place are exposed to higher risks of misconnections.22
To reduce the risk of wrong route medication errors the International Organization for Standardization (ISO) has developed standards for small-bore connectors, one being in the field of Neuraxial and Regional Anesthesia (ISO 80369-6). Devices that comply with the ISO 80369-6 standard are called NRFit®. They are 20 % smaller in diameter compared to Luer connectors and come with a yellow colour coding.23
Checklists for Healthcare Workers
Risk: Wrong medication
Antineoplastic agents are mostly classified as high-alert medications. These are medications that pose an increased risk of patient harm when involved in medication errors.24
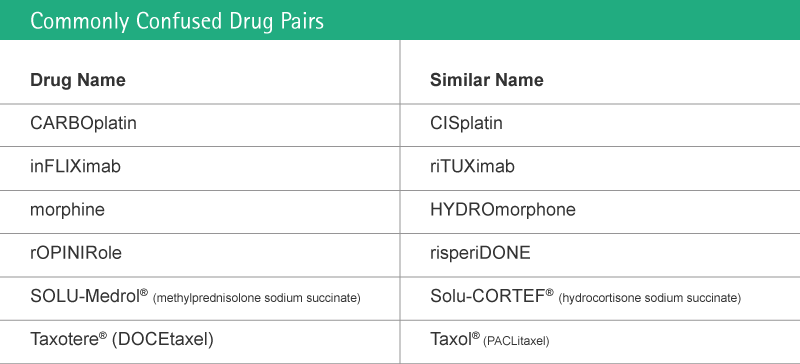
A majority of wrong medication errors derives from prescriptions, one of the causes being sound-alike drugs (name similarity).
Safeguards to avoid wrong medication errors
The U.S. Food and Drug Administration 2001 in their “Name Differentiation Project” requested manufacturers of sixteen look-alike name pairs to voluntarily revise the appearance of their established names in order to minimize medication errors resulting from look-alike confusion.
The encouraged manufacturers to use revised labels and labeling that visually differentiated their established names with the use of "Tall Man" (bolded uppercase) letters.25
The list has been adopted and enlarged by the Institute of Safe Medication Practises26, including but is not limited to antineoplastic agents.
Order verification by double checking the form should be a prerequisite before drugs are prepared by the pharmacist.27
Cheat Sheet
Cost of medication error in chemotherapy
Errors are also costly in terms of opportunity costs. Dollars spent on having to repeat diagnostic tests or counteract adverse drug events are dollars unavailable for other purposes. Purchasers and patients pay for errors when insurance costs and copayments are inflated by services that would not have been necessary had proper care been provided. It is impossible for the nation to achieve the greatest value possible from the billions of dollars spent on medical care if the care contains errors.
But not all the costs can be directly measured. Errors are also costly in terms of loss of trust in the system by patients and diminished satisfaction by both patients and health professionals. Patients who experience a longer hospital stay or disability as a result of errors pay with physical and psychological discomfort. Healthcare professionals pay with loss of morale and frustration at not being able to provide the best care possible and society, in general, pay in terms of lost worker productivity, reduced school attendance by children, and lower levels of population health status.28
Learn more
Preventive Strategies
„Primum nil nocere. (First do no harm.)“
“It may be part of human nature to err, but it is also part of human nature to create solutions, find better alternatives and meet the challenges ahead”. On their landmark publication in 1999, Kohn and colleagues of Institute of Medicine (IOM) called the entire healthcare sector to action.28 The IOM panel called for a transformation in the way health-care professionals understand medical error by applying principles from cognitive psychology and human factors, the study of human performance in work environments. Improvements in aviation and other safety-oriented industries, such as chemical engineering, manufacturing, and nuclear power, showed that complex systems, rather than individual practitioners, were the primary sources of error and a target for improvement opportunities through simplification, standardization, and technology.30
This has in the same year been called “Swiss Cheese Model”, referring to multiple layers of safety shields to prevent errors (holes in the cheese) to reach the patient.29 This model has been the conceptual foundation for the development of Critical Incident Reporting Systems for the reporting of and learning from critical incidents and near misses.
The American Society of Hospital Pharmacists (ASHP) consequentially developed a guideline on Preventing Medication Errors with Chemotherapy and Biotherapy in 2002 which has recently been updated.32 The guideline comprises recommendations for healthcare organizations, for multidisciplinary monitoring of medication use and verification, for prescribing systems and prescribers, for medication preparation and dispensing systems and roles for pharmacists, for medication administration systems and roles for nurses, for patient education, for manufacturers and regulatory agencies, and recommendations for identifying and managing medication errors.
Checklists for Healthcare Workers
Risk: Wrong administration technique
Wrong administration techniques may comprise multiple aspects of the infusion. One example is discussed in the following:
„Paclitaxel is a chemotherapeutic drug frequently used for breast, ovarial and bronchial cancer. The drug is likely to form microbubbles and particulate matter. The suppliers recommend that an in-line IV filter should be used during the infusion of the agent (SmpC Paclitaxel). Not using the inline filter might result in particles being infused into the patient [33].“
Particles arising from infusion therapy may induce or aggravate inflammatory response syndromes. They have been shown to generate thrombosis, impair microcirculation, and modulate immune response. Sources of particles include components of infusion systems, incomplete reconstitution of solutions or drug incompatibility reactions. Up to one million particles may be infused per patient per day. In-line filters incorporated into infusion lines retain particles and thereby nearly entirely prevent their infusion.33
Others would be errors in assembling giving sets for secondary infusions with or without pumps, Luer access-devices unintentionally left open after use or needlestick injury due to needle-based manipulation.
Risk: Wrong Patient
Incidents involving patient misidentification (e.g., “wrong patient” errors) are not uncommon in oncology practice. Many of these incidents are near-miss or close-call situations that are averted at some point prior to reaching the patient. Patient misidentification is under-reported and its incidence is unknown.34
Case Study
-
-
Wrong Patient
A nurse asked Mrs. Jackson to come back to the treatment room of an oncologist’s office for chemotherapy ...[33]
Prevention of "wrong patient" errors
In 2002, the Joint Commission created its National Patient Safety Goals program. The No. 1 priority: improving the accuracy of patient identification. To meet this goal, healthcare providers use at least two patient identifiers — usually, name and date of birth. American Society of Clinical Oncology (ASCO) and the Oncology Nursing Society collaborated to create chemotherapy administration safety standards to reduce the risk of error when providing adult patients with chemotherapy and give a framework for best practices in cancer care.35
At a glance:34
- Patient misidentification can occur at virtually any point in a patient encounter
- Safety systems have the potential to reduce, but not eliminate, “wrong patient” errors because patient care is vulnerable to human error
- Proper patient identification begins with patient registration
Products that can help to prevent medication errors
Products that can help preventing medication errors
References
- Makary M.A. Daniel M (2016): Medical error - the third leading cause of death in the US.BMJ 2016; 353.
- Richard A. Know (1995): Doctor’s order killed cancer patient; in: The Boston Globe, Nr. 82, Vol. 247, p.1.
- The Boston Globe, 2004
- National Coordinating Council for Medical Error Reporting and Prevention (NCCMERP): What is a Medication Error. available at: https://www.nccmerp.org/about-medication-errors; accessed 05-28-2019
- Fortescue, E. B., et al: Prioritizing Strategies for Preventing Medication Errors and Adverse Drug Events. Pediatric Inpatients. Pediatrics, 2003; 111(4); 722-729. available at: http://dx.doi.org/10.1542/peds.111.4.722; accessed 05-28-2019
- Ford et al (2006): Study of Medication Errors on a Community Hospital Oncology Ward. Journal of Oncology Practice, 2006, 2 (4), 149-154. available at: https://ascopubs.org/doi/full/10.1200/jop.2006.2.4.149; accessed 05-06-2019.
- Lustig A. (2000): Medication error prevention by pharmacists – an Israeli solution. Pharmacy World and Science. 2000, 22 (1), 21–25.
- Schulmeister L. (1999): Chemotherapy medication errors: descriptions, severity, and contributing factors. Oncol Nurs Forum. 1999; 26(6):1033-42.
- Phillips J. et al. (2001): Retrospective analysis of mortalities associated with medication errors. American Journal of Health-System Pharmacy. 2001. 58 (19), 1835–1841. available at: https://doi.org/10.1093/ajhp/58.19.1835; accessed 06-07-2019
- ABC. (2015): South Australian Government launches inquiry over chemotherapy drug-dosing bungle. [online] available at: http://www.abc.net.au/news/2015-08-05/sa-government-launches-inquiry-over-chemotherapy- ungle/6673890
- MacIennan, L. (2016): Chemotherapy bungle at Adelaide hospitals due to clinical failures, SA Health Minister says. [online] available at: http://www.abc.net.au/news/2016-02-09/chemotherapy-bungle-at-adelaide-hospitals-under-review/7153168 accessed: 06-07-2019
- Platzhalter Chemotherapy Orders
- Rooker JC, Gorard DA (2007): Errors of intravenous fluid infusion rates in medical inpatients. Clin Med. 2007;7: 482–5. available at: https://pdfs.semanticscholar.org/ec0d/acd06790eef073fb64a0678b74ca065e0516.pdf; accessed: 06-07-2019
- Hennipmann B. et al (2009): Intrathecal Vincristine. 3 Fatal Cases and a Review of the Literature. Journal Pediatric Hematol Oncol. 2009, 31 (11), 816-819.
- Schulmeister L. (2006): Look-alike, sound-alike oncology medications. Clin J Oncol Nurs 2006, 10(1):35-41.
- Bates DW et al (1995): Relationship between medication errors and adverse drug events. J Gen Intern Med 1995;10 (4):199-205
- Noble D. (2010): The quest to eliminate intrathecal vinchristine errors: a 40-year journey. BMJ Quality & Safety 2010, 19, 323-326.
- Toft B (2001): External Inquiry into the adverse incident that occurred at Queen’s Medical Centre, Nottingham, 4th January 2001, [online]. Available at: www.who.int/patientsafety/news/Queens%20Medical%20Centre%20report%20(Toft).pdf accessed: 06-07-2019
- Arzneimittelkommission der deutschen Ärzteschaft (2005): Vincristin: Toedliche Zwischenfaelle nach versehentlicher intrathekaler Gabe. Deutsches Aerzteblatt 2005, 102,1615.
- Dyer c (2001): Doctors suspended after injecting wrong drug into spine. BMJ 2001, 322 (7281). 257.
- Kress R. et al. (2016): Unintentional Infusion of Phenylephrine into the Epidural Space. A&A Case Rep. 2016, 6(5),124-7.
- International Organization for Standardization (2016): Small bore connectors for liquids and gases in healthcare applications -- Part 6: Connectors for neuraxial applications. [online] available at: https://www.iso.org/standard/50734.html accessed: 06-07-2019.
- Institute for Safe Medication Practices (2014): ISMP List of High-Alert Medications in Acute Care Settings [online] available at: https://www.ismp.org/sites/default/files/attachments/2018-01/highalertmedications%281%29.pdf accessed 06-07-2019
- U.S. Food & Drug Administration (2001): Name Differention Project [online] available at: https://www.fda.gov/Drugs/DrugSafety/MedicationErrors/ucm164587.htm accessed: 06-07-2019
- Institute for Safe Medication Practices (2016): FDA and ISMP Lists of Look-Alike Drug Names with Recommended Tall Man Letters [online] available at: https://www.ismp.org/sites/default/files/attachments/2017-11/tallmanletters.pdf accessed: 06-07-2019
- Erdlenbruch B (2002): Chemotherapy errors in oncology. Med Pediatr Oncol 2002, 38, 353-356. Available at: https://doi.org/10.1002/mpo.1344 accessed: 06-07-2019
- Linda T. Kohn L.T. (2000): To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press.
- Ranchon et al. (2011): Chemotherapeutic errors in hospitalised cancer patients: attributable damage and extra costs. BMC Cancer 2011, 11:478.
- Weingart SN (2018): Chemotherapy medication errors. Lancet Oncol 2018, 19 (4), 191–99.
- Reason, James (2000-). Human error: models and management. BMJ, 320 (7237): 768–770.
- Goldspiel B et al. (2015): ASHP guidelines on preventing medication errors with chemotherapy and biotherapy. American journal of health-system pharmacy, 2015, 72, 6–35.
- Sasse M. et al. (2015): In-line Filtration Decreases Systemic Inflammatory Response Syndrome, Renal and Hematologic Dysfunction in Pediatric Cardiac Intensive Care Patients. Pediatric Cardiology 2015, 36 (6),1270–1278.
- Schulmeister L (2007): Patient Misidentification in Oncology. Clinical Journal of Oncology Nursing 2007, 12 (3), 495-498. Available at: https://cjon.ons.org/cjon/12/3/patient-misidentification-oncology-care accessed: 06-07-2019
- Schulmeister L (2002): Searching for Information for Presentations and Publications. Clinical Nurse Specialist, 2002, 16 (2); 79-84